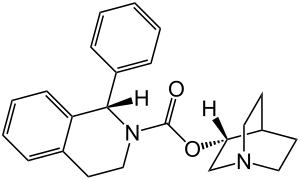
Solifenacin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structural formula of solifenacin | |||||||||||||
| General | |||||||||||||
| Non-proprietary name | Solifenacin | ||||||||||||
| other names |
(1 S , 3 ' R ) -1-Azabicyclo [2.2.2] oct-8-yl-1-phenyl-3,4-dihydro-1 H -isoquinoline-2-carboxylate |
||||||||||||
| Molecular formula |
|
||||||||||||
| Brief description |
colorless, slightly yellowish crystals (solifenacin succinate) |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Melting point |
approx. 143 ° C (solifenacin succinate) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Solifenacin is the generic name of a drug used to treat symptoms of overactive bladder (Engl. "Overactive bladder", "OAB"). It belongs to the pharmacological group of urological spasmolytics .
Solifenacin is a yellow oil. Its salt of succinic acid (solifenacin succinate ) is used medicinally .
pharmacology
Applications and clinical effects
Solifenacin is approved for the symptomatic treatment of urge incontinence or imperative urination and pollakiuria that can occur in patients with overactive bladder syndrome .
Mechanism of action
The urinary bladder is innervated by parasympathetic , cholinergic nerves . Acetylcholine causes a contraction of the smooth muscles of the detrusor muscle via muscarinic receptors , mainly via the subtype M 3 . As a receptor antagonist , solifenacin inhibits the muscarinic receptor M 3 competitively and specifically, since it has little or no affinity for various other receptors or ion channels .
Solifenacin binds about twelve times better to the human M 3 than to the M 2 muscarinic receptor. The ratio to M 1 receptors is about 2.5. These results from animal experiments cannot be transferred to humans.
Side effects
Due to the pharmacological action of solifenacin, anticholinergic side effects of usually mild to moderate severity can be produced. The frequency is dose-dependent.
The most frequently described side effect, the incidence of which is significantly lower than that of other anticholinergics , is dry mouth , which occurs in about 11% of patients with a dose of 5 mg solifenacin daily. In addition, side effects such as B. constipation , nausea, abdominal pain, blurred vision and others.
In a clinical study, solifenacin (both approved doses were evaluated together) was compared directly with a retarded anticholinergic. More patients reported dry mouth with solifenacin than with the comparator (30% versus 24%). The same is true of constipation (6.4% versus 2.5%). Visual disturbances were less common with solifenacin (0.7% versus 1.7%).
Interactions
There are no known clinically relevant drug interactions with solifenacin.
Trade names
Vesicare (A, CH), Vesicare (D)
Individual evidence
- ↑ a b c The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 1494, ISBN 978-0-911910-00-1 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ External identifiers or database links for solifenacin succinate : CAS number: 242478-38-2, EC number: 620-505-5, ECHA InfoCard: 100.149.369 , PubChem : 216457 , ChemSpider : 187603 , Wikidata : Q374826 .
- ^ SS Hegde et al .: "Antimuscarinics for the treatment of overactive bladder: current options and emerging therapies". Current Opinion in Investigational Drugs Vol 5 (2004), pp. 40-49. PMID 14983972 .
- ↑ CJ Kelleher, L. Cardozo, CR Chapple, F. Haab, AM Ridder: Improved quality of life in patients with overactive bladder symptoms treated with solifenacin. In: BJU international. Volume 95, Number 1, January 2005, pp. 81-85, doi : 10.1111 / j.1464-410X.2004.05255.x , PMID 15638900 .