Tetrachlorophthalic anhydride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
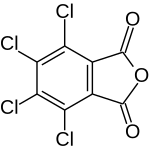
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetrachlorophthalic anhydride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 Cl 4 O 3 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 285.90 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.98 g cm −3 |
|||||||||||||||
| Melting point |
253-256 ° C |
|||||||||||||||
| boiling point |
350 ° C |
|||||||||||||||
| solubility |
practically insoluble in water (0.8 mg l −1 at 21 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tetrachlorophthalic anhydride is a chemical compound from the group of substituted carboxylic acid anhydrides .
Extraction and presentation
Tetrachlorophthalic anhydride can be prepared by the direct chlorination of phthalic anhydride in an organic solvent . If the chlorination is carried out in fuming sulfuric acid with iodine as the catalyst , the intermediate product 3,6-dichlorophthalic anhydride is formed.
properties
Tetrachlorophthalic anhydride is a flammable colorless solid that is in the form of prisms or needles. It is practically insoluble in cold water and changes to tetrachlorophthalic acid in hot water. The reaction of tetrachlorophthalic anhydride with amines results in tetrachlorophthalimides , which serve as protective groups for amines in carbohydrate and amino acid chemistry.
use
Tetrachlorophthalic anhydride is used as a flame retardant additive in paints. It is a raw material for the production of tetrachlorophthalate resins, fungicides , rubber, plasticizers , non-flammable waxes, plastics and lubricants . It is also a curing catalyst for casting compounds made from amino resins and epoxy resins . It is also used to produce phthalocyanines , xanthene dyes, isoindolinone or quinophthalone pigments and is also used in color filters for liquid crystal displays , in lithographic printing plates and for the production of tetrafluorinated phthalic and benzoic acid derivatives.
Individual evidence
- ↑ Åke Bergman , Andreas Rydén, Robin J. Law, Jacob de Boer, Adrian Covaci, Mehran Alaee, Linda Birnbaum, Myrto Petreas, Martin Rose, Shinichi Sakai, Nele Van den Eede, Ike van der Veen: A novel abbreviation standard for organobromine , organochlorine and organophosphorus flame retardants and some characteristics of the chemicals . In: Environment International . tape 49 , 2012, p. 57–82 , doi : 10.1016 / j.envint.2012.08.003 , PMC 3483428 (free full text).
- ↑ a b c d e f g h i j k Entry on tetrachlorophthalic anhydride in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on tetrachlorophthalic anhydride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b c d Entry on tetrachlorophthalic anhydride. In: Römpp Online . Georg Thieme Verlag, accessed on September 3, 2015.
- ↑ Hubert Suter : Phthalic anhydride and its use - plasticizers, polyester resins, dyes, intermediate products . Springer-Verlag, 1972, ISBN 978-3-642-51103-5 , p. 14 ( limited preview in Google Book search).
- ↑ Paul Heinz List, Ludwig Hörhammer: Chemicals and Drugs Part C: T-Z . 4th edition. Springer-Verlag, 1979, ISBN 978-3-642-67085-5 , pp. 57 ( limited preview in Google Book search).


