Thiamphenicol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
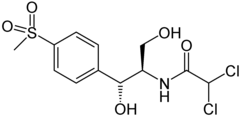
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Thiamphenicol | |||||||||||||||||||||
| other names |
(1 R , 2 R ) -2,2-dichloro- N - [1,3-dihydroxy-1- (4-methylsulfonylphenyl) propan-2-yl] acetamide |
|||||||||||||||||||||
| Molecular formula | C 12 H 15 Cl 2 NO 5 S | |||||||||||||||||||||
| Brief description |
crystalline powder or crystals, fine, white to yellowish white |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 356.22 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
164.3-166.3 ° C |
|||||||||||||||||||||
| solubility |
slightly soluble in water, very slightly soluble in dimethylacetamide , slightly soluble in acetonitrile and dimethylformamide , soluble in methanol , slightly soluble in acetone and ethanol , slightly soluble in ethyl acetate |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Thiamphenicol is an antibiotic . It is the methyl - sulphonyl analogue of chloramphenicol and also has a similar spectrum of activity, however, is 2.5 to 5 times as potent. Like chloramphenicol, it is insoluble in water, but very soluble in lipids . It is used in veterinary medicine in many countries, but it is also approved for humans in China, Italy and Turkey. Its main advantage over chloramphenicol is that it has never been associated with aplastic anemia .
The chemical synthesis of thiamphenocol is described in the literature. Florfenicol is a fluorinated analog .
literature
- Raymond J, Boutros N, Bergeret M: Role of thiamphenicol in the treatment of community-acquired lung infections . In: Med Trop (Mars) . 64, No. 1, 2004, pp. 33-38. PMID 15224555 .
- Marchese A, Debbia E, Tonoli E, Gualco L, Schito A: In vitro activity of thiamphenicol against multiresistant Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus in Italy . In: J Chemother . 14, No. 6, 2002, pp. 554-561. PMID 12583545 .
Individual evidence
- ↑ a b c data sheet THIAMPHENICOL CRS (PDF) at EDQM , accessed on November 8, 2009.
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 1599, ISBN 978-0-911910-00-1 .
- ↑ Thiamphenicol data sheet from Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher, Dieter Reichert: Pharmaceutical Substances . 4th edition, Thieme-Verlag, Stuttgart 2000. S. 2016–2017. ISBN 978-1-58890-031-9 .