Tolclofos-methyl
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
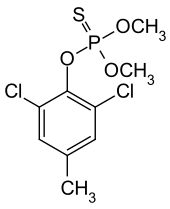
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tolclofos-methyl | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 11 Cl 2 O 3 PS | |||||||||||||||
| Brief description |
colorless solid with a faint characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 301.13 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.838 g cm −3 |
|||||||||||||||
| Melting point |
78-80 ° C |
|||||||||||||||
| boiling point |
120 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tolclofos-methyl is a chemical compound from the group of thiophosphoric acid esters .
Extraction and presentation
Tolclofos-methyl can be obtained by reacting 2,6-dichloro-4-methylphenol with dimethylthiophosphoryl chloride or by reacting 2,6-dichloro-4-methylphenol with thiophosphoryl trichloride and then with methanol .
properties
Tolclofos-methyl is a colorless solid with a faint characteristic odor, which is practically insoluble in water.
use
Tolclofos-methyl is used as an active ingredient in pesticides. It is an organophosphorus fungicide used to control soil diseases caused by Rhizoctonia solani , Corticium rolfsii , Typhula incarnata and Typhula ishikariensis . The effect is due to inhibition of mycelial growth by inhibiting phospholipid - biosynthesis .
Admission
It has been approved in Germany since 1983.
Tolclofos-methyl was approved for use as a fungicide in the European Union with effect from February 1, 2012. In Germany, Austria and Switzerland, plant protection products with this active ingredient are approved.
proof
In addition to gas and liquid chromatography , tolclofos-methyl can also be detected using immunoassay methods.
Individual evidence
- ↑ a b c d e f g h Entry for CAS no. 57018-04-9 in the GESTIS substance database of the IFA , accessed on September 4, 2012(JavaScript required) .
- ↑ a b c d e FAO: TOLCLOFOS-METHYL (PDF; 266 kB)
- ↑ Entry on Tolclofos-methyl in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on August 1, 2013.
- ↑ Entry on O- (2,6-dichloro-p-tolyl) O, O-dimethyl thiophosphate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can use the harmonized classification and labeling expand .
- ↑ Tolclofos-methyl data sheet from Sigma-Aldrich , accessed on May 21, 2017 ( PDF ).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 336 ( limited preview in Google Book search).
- ↑ Terry R. Roberts, David H. Hutson, Philip W. Lee, Peter H. Nicholls: Metabolic Pathways of Agrochemicals: Part 2: Insecticides and Fungicides . Royal Society of Chemistry, 1999, ISBN 0-85404-499-X , pp. 1259 ( limited preview in Google Book Search).
- ↑ Peter Brandt: Reports on Plant Protection Products 2009: Active Ingredients in Plant Protection Products ; Approval history and regulations of the Plant Protection Application Ordinance . Springer DE, 2010, ISBN 3-0348-0028-2 , pp. 27 ( limited preview in Google Book search).
- ↑ Directive 2006/39 / EC of the Commission of April 12, 2006 amending Directive 91/414 / EEC of the Council to include the active substances clodinafop, pirimicarb, rimsulfuron, tolclofos-methyl and triticonazole (PDF)
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Tolclofos-methyl in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 13, 2016.
- ↑ ZK Li, YY Zhu, XG Yin, CF Peng, W. Chen, LQ Liu, LM Yin, CL Xu: Development of an indirect enzyme-linked immunosorbent assay for the organophosphorus pesticide paraoxon-methyl. In: Immunological investigations. Volume 38, Number 6, 2009, pp. 510-525. PMID 19811409 .


