Triasulfuron
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
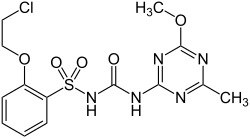
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Triasulfuron | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 14 H 16 ClN 5 O 5 S | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 401.83 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.47 g cm −3 |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| Vapor pressure |
0.0021 mPa (25 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Triasulfuron is a chemical compound from the group of sulfonylureas , which was introduced by Ciba-Geigy in 1985 .
Extraction and presentation
Triasulfuron can be prepared by a multi-stage reaction starting from o -aminophenol . This reacts with sodium nitrite to form the diazonium salt , which further reacts with sulfur dioxide in the presence of copper (I) chloride , with ammonium hydroxide , 2-chloroethyl p -toluenesulfonate and phosgene . With 2-amino-4-methoxy-6-methyl-1,3,5-triazine, the end product is now formed.
use
Triasulfuron is used as a selective post-emergence herbicide in grain cultivation. The effect is based on the inhibition of acetolactate synthase .
Admission
The approval of plant protection products with this active ingredient was revoked in Germany on September 30, 2016. After the revocation, there was a sell-off period for stocks until March 30, 2017 and a use-by period until September 30, 2017.
In the other EU countries and Switzerland, there is no approval for plant protection products with this active ingredient.
Individual evidence
- ↑ a b c d e Entry on Triasulfuron in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on March 4, 2014.
- ↑ a b Entry on Triasulfuron. In: Römpp Online . Georg Thieme Verlag, accessed on March 4, 2014.
- ↑ a b Data sheet Triasulfuron at Sigma-Aldrich , accessed on May 20, 2017 ( PDF ).
- ↑ Entry on 1- [2- (2-chloroethoxy) phenylsulfonyl] -3- (4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA ), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b Entry on triasulfuron in the GESTIS substance database of the IFA , accessed on March 4, 2014 (JavaScript required)
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 202 ( limited preview in Google Book search).
- ↑ Gerrit Hogrefe: Important building blocks in the herbicide range are missing . Ed .: agrarzeitung. July 15, 2016, p. 18 .
- ↑ bvl.bund.de: BVL - Fachmachrichten - Approvals of pesticides with isoproturon and triasulfuron will be revoked on September 30, 2016 , accessed on July 23, 2016.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on triasulfuron in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on December 6, 2019.
