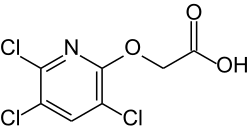
Triclopyr
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Triclopyr | ||||||||||||||||||
| other names |
[(3,5,6-Trichloropyridin-2-yl) oxy] acetic acid |
||||||||||||||||||
| Molecular formula | C 7 H 4 Cl 3 NO 3 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 256.5 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.85 g cm −3 |
||||||||||||||||||
| Melting point |
150.5 ° C |
||||||||||||||||||
| solubility |
heavy in water (8.1 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Triclopyr is a chemical compound from the group of pyridinecarboxylic acids . It is the pyridine analogue of 2,4,5-trichlorophenoxyacetic acid .
properties
Triclopyr is a white solid that is moderately soluble in water. It is stable to hydrolysis .
synthesis
The synthesis of Triclopyr starts from pyridine , which is chlorinated to pentachloropyridine . The chlorine atom at the para position is removed with hydrazine . The 2,3,5,6-tetrachloropyridine reacts with potassium cyanide or sodium cyanide and trioxane to form the last intermediate product, which is carboxylated with hydrogen chloride to give triclopyr .
use
Triclopyr is used as an active ingredient in crop protection products. It is a systemic and selective herbicide from the class of growth substances.
Admission
Effective June 1, 2007, the EU Commission added Triclopyr to the list of permissible active ingredients in pesticides for use as a herbicide .
In a number of EU countries, including Germany and Austria, as well as Switzerland, plant protection products with this active ingredient are approved.
Individual evidence
- ↑ a b c d e f g h i Entry on Triclopyr in the GESTIS substance database of the IFA , accessed on February 19, 2018(JavaScript required) .
- ↑ Triclopyr data sheet at Sigma-Aldrich , accessed on May 20, 2017 ( PDF ).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , Triclopyr, pp. 540-541 ( limited preview in Google Book search).
- ↑ Directive 2006/74 / EC of the Commission of August 21, 2006 (PDF) amending Council Directive 91/414 / EEC to include the active substances dichlorprop-P, metconazole, pyrimethanil and triclopyr.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Triclopyr in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on December 8, 2019.
