Triphenyl tetrazolium chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Triphenyl tetrazolium chloride | |||||||||||||||
| other names |
2,3,5-triphenyl-2 H -tetrazolium chloride |
|||||||||||||||
| Molecular formula | C 19 H 15 ClN 4 | |||||||||||||||
| Brief description |
almost colorless fine crystalline powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 334.81 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
250 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tetrazolium chloride ( TTC ), more specifically 2,3,5-triphenyltetrazolium chloride , a quaternary ammonium compound and a redox - dye .
properties
Triphenyl tetrazolium chloride is a colorless, water-soluble redox indicator . The color change occurs with a redox reaction :
- In the oxidized state (tetrazolium) the indicator is colorless .
- In the reduced state (formazan) the indicator is red . Its extinction maximum is 492 nm.
Reactions
The water-soluble, colorless 2,3,5-triphenyltetrazolium chloride is reduced to the water-insoluble red dye 1,3,5-triphenylformazan , taking up two electrons (e - ) and one proton (H + ) . The reduction takes place through a double single electron transfer (SET):
The tetrazolium cation (T + ) is reduced to formazan (F) via a tetrazolium radical (T ·) .
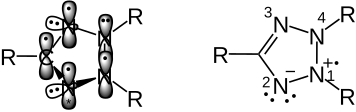
Limiting formulas of the structure of the tetrazolium radical
Possible resonance structures of the intermediate radical can be derived from a classical perspective:
The initial situation of the triphenyltetrazolium cation can be seen on the left, the classic view of the electron distribution on the right. Tetrazole is an aromatic heterocycle. At N 1, 2, 3, 4 and C there is sp² hybridization , the atoms each have a singly or, in the case of N 4, doubly occupied p-atom orbital. The overlap of the five parallel p orbitals leads to the formation of the aromatic π-electron system. The single electron transfer can take place in the singly occupied p orbitals at N 1, 2, 3 . N 2 and N 3 carry lone pairs of electrons in one of their sp 2 -HOs, which are not involved in the aromatic system.
If the SET takes place in the p orbital of N 1 (symbolized with "*") the following picture results:
classical mesomerism is shown on the right.
If the SET takes place in the sp 2 orbital of N 2 or 3 (symbolized with "*"), the following picture results for N 2 :
"Classical" mesomerism is not possible here. An unlikely nitrene radical structure would result in both cases (analogous for N 3 ).
use
Triphenyltetrazolium chloride is used as a TTC test as is the analogous 3- (1-naphthyl) derivative (tetrazolium violet), tetrazolium blue and tetrazol purple for the detection of intact cells ( vital staining ). The tetrazolium cation is reduced to formazan by dehydrogenases in the respiratory chain (primarily from complex I ).
It is used in histochemistry for measuring the activity of dehydrogenases, for measuring the germination capacity of seeds, for rapid tests for determining the value of antibiotics and disinfectants, for determining the quality of activated sludge and in food chemistry as an indicator for the bacterial content of milk.
In thin-layer chromatography it is suitable as a spray reagent for reducing sugars, corticosteroids and other reducing agents .
Other tetrazolium compounds
- Mono-tetrazolium salts
- INT ( I od n itro t etrazolium chloride)
- MTT (thiazolyl blue; 3- (4,5-di m ethyl-2- t hiazolyl) -2,5-diphenyl-2 H - t etrazoliumbromid)
- XTT (2,3-bis (2-methoxy-4-nitro-5-sulfophenyl) -5 - ((phenylamino) carbonyl) -2 H -tetrazolium hydroxide)
- MTS (3- (4,5-dimethylthiazol-2-yl) -5- (3-carboxymethoxyphenyl) -2- (4-sulfophenyl) -2 H -tetrazolium)
- WST-1 (2- (4-iodophenyl) -3- (4-nitrophenyl) -5- (2,4-disulfophenyl) -2 H -tetrazolium monosodium salt)
- Di-tetrazolium salts
- BT ( T etrazolium- B lue)
- NBT ( N itro b lau- T etrazolium chloride )
- TNBT ( T etra n itro t etrazolium- B lue)
See also
Individual evidence
- ↑ a b c d e Entry on 2,3,5-triphenyl-2H-tetrazolium chloride. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b Data sheet 2,3,5-Triphenyltetrazolium chloride from Sigma-Aldrich , accessed on October 22, 2016 ( PDF ).
- ↑ W. Ried: Formazans and tetrazolium salts, their syntheses and their importance as reduction indicators and vital dyes. In: Angewandte Chemie . Vol. 64, 1952, pp. 391-396, doi : 10.1002 / anie.19520641403 .
- ↑ Monica C. Gonzilez, Enrique San Romàn: Photochemistry of Aqueous Solutions of Triphenyltetrazolium Chloride. In: J. Phys. Chem. 93 (1989), pp. 3536-3540.
- ↑ FP Altman: tetrazolium salts and formazan. In: Prog. Histochem. Cytochem. Vol. 9 (1976), pp. 1-56, PMID 792958 .
- ↑ M. Ogur et al .: Tetrazolium overlay technique for population studies of respiration deficiency in yeast. In: Science. 125: 928-929 (1957), PMID 13421693 .
- ↑ PR Rich et al .: The sites of interaction of triphenyltetrazolium chloride with mitochondrial respiratory chains. In: FEMS Microbiol. Lett. 202: 181-187 (2001), PMID 11520612 .
- ↑ External identifiers or database links for iodonitrotetrazolium chloride : CAS number: 146-68-9, EC number: 205-676-2, ECHA InfoCard: 100.005.161 , PubChem : 64957 , ChemSpider : 58482 , Wikidata : Q15411024 .
- ↑ External identifiers of or database links to 3- (4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide: CAS number: 298-93-1, EC number: 206-069-5, ECHA InfoCard: 100.005.518 , PubChem : 64965 , ChemSpider : 58490 , Wikidata : Q27124034 .
- ↑ External identifiers of or database links for 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl) -5 - ((phenylamino) carbonyl) -2H-tetrazolium hydroxide : CAS number: 117038-70- 7, PubChem : 5704 , Wikidata : Q83018206 .
- ↑ a b c C. McCluskey et al: An evaluation of three new-generation tetrazolium salts for the measurement of respiratory activity in activated sludge microorganisms. In: Microb. Ecol. Vol. 49 (2005), pp. 379-387, PMID 16003480 doi : 10.1007 / s00248-004-0012-z .
- ↑ External identifiers of or database links for 3- (4,5-Dimethylthiazol-2-yl) -5- (3-carboxymethoxyphenyl) -2- (4-sulfophenyl) -2H-tetrazolium : CAS number: 138169- 43-4, Wikidata : Q83028878 .
- ↑ External identifiers of or database links for 2- (4-iodophenyl) -3- (4-nitrophenyl) -5- (2,4-disulfophenyl) -2H-tetrazolium monosodium salt : CAS number: 150849-52- 8, EC number: 604-753-1, ECHA InfoCard: 100.128.588 , Wikidata : Q72484399 .
- ↑ External identifiers or database links for tetrazolium blue : CAS number: 1871-22-3, EC number: 217-488-8, ECHA InfoCard: 100.015.899 , PubChem : 9853362 , ChemSpider : 8029073 , Wikidata : Q27145163 .
- ↑ External identifiers of or database links for nitro blue tetrazolium chloride : CAS number: 1184-43-6, EC number: 214-665-1, ECHA InfoCard: 100.013.332 , Wikidata : Q72507820 .