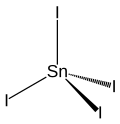
Tin (IV) iodide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| Wedges to clarify the spatial structure | |||||||||||||||||||
| Space group |
Pa 3 (No. 205) |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tin (IV) iodide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | SnI 4 | ||||||||||||||||||
| Brief description |
Orange solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 626.328 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
4.47 g cm −3 |
||||||||||||||||||
| Melting point |
144.5 ° C |
||||||||||||||||||
| boiling point |
364.5 ° C |
||||||||||||||||||
| solubility | |||||||||||||||||||
| Refractive index |
2,106 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Tin (IV) iodide is an inorganic chemical compound of tin from the group of iodides .
Extraction and presentation
Tin (IV) iodide can be obtained by direct synthesis from the elements or by heating a tin (II) chloride solution with iodine.
properties
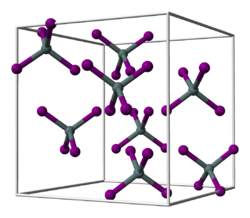
Tin (IV) iodide is an orange solid that hydrolyzes in water . It has a cubic crystal structure with the space group Pa 3 (space group no. 205) , the lattice parameter a = 1226 pm and eight formula units per unit cell . This corresponds to a cubic close packing of iodine atoms, in which 1/8 of all tetrahedral gaps are occupied by tin atoms. This leads to discrete tetrahedral SnI 4 molecules.
Individual evidence
- ↑ a b c d e Moeller, T., Edwards, DC, Brandt, RL and Kleinberg, J .: Tin (IV) Iodide (Stannic Iodide) . In: Inorganic Syntheses . 4, 1953, pp. 119-121. doi : 10.1002 / 9780470132357.ch40 . ( limited preview in Google Book search)
- ↑ a b c d data sheet Tin (IV) iodide (PDF) from Strem, accessed on December 25, 2012.
- ↑ a b c data sheet Tin (IV) iodide, anhydrous, powder, 99.999% trace metals basis from Sigma-Aldrich , accessed on September 9, 2011 ( PDF ).
- ^ D'Ans-Lax, paperback for chemists and physicists. Vol. 3 (1998) ISBN 3-540-60035-3 , p. 740.
- ↑ Hickling, GG: Gravimetric analysis: The synthesis of tin iodide . In: J. Chem. Educ. . 67, No. 8, 1990, pp. 702-703. doi : 10.1021 / ed067p702 .
- ↑ F. Meller and I. Fankuchen: The crystal structure of tin tetraiodide . In: Acta Crystallographica . 8, 1955, pp. 343-344. doi : 10.1107 / S0365110X55001035 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 970.