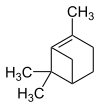
Pinene
Pinene (stress on the second syllable: Pin e n ) are monoterpene - hydrocarbons , colorless liquids with the empirical formula C 10 H 16 . Pinene are components of essential oils .
Representative
There are six pinene isomers , two each of α-pinene , two β-pinene and two known δ-pinene e; the latter are described in the literature as (+) - cis -δ-pinene and (-) - cis -δ-pinene.
In 1907 Otto Wallach assigned three pinene as α, β- and γ-pinene. Another representative was discovered in 1921 and consequently referred to as δ-pinene. The constitution of "γ-pinene" assigned by Wallach was rejected again by Harry Schmidt in 1947 because it contradicted Bredt's rule . The above-mentioned classic designations of the pinene (α, β, γ, δ) were retained because they had already become established in the literature at that time.
| Pinene | ||||||||||||||||
| Surname | (+) - α-pinene | (-) - α-pinene | (+) - β-pinene | (-) - β-pinene | (+) - cis -δ-pinene | (-) - cis -δ-pinene | ||||||||||
| other names | Pin-2 (3) -ene 2-pinene 2,6,6-trimethylbicyclo- [3.1.1] hept-2-ene |
Pin-2 (10) -en 2 (10) -pinene nopine pseudopine 6,6-dimethyl-2-methylenebicyclo- [3.1.1] heptane |
||||||||||||||
| Structural formula |

|
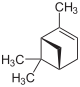
|

|

|

|

|
||||||||||
| CAS number | 7785-70-8 | 7785-26-4 | 19902-08-0 | 18172-67-3 | ||||||||||||
| 2437-95-8 (±) 80-56-8 (unspecified) |
23089-32-9 (±) 127-91-3 (unspecified) |
|||||||||||||||
| PubChem | 82227 | 440968 | 10290825 | 440967 | 12314302 | |||||||||||
| Molecular formula | C 10 H 16 | |||||||||||||||
| Molar mass | 136.24 g mol −1 | |||||||||||||||
| Physical state | liquid | |||||||||||||||
| Brief description | colorless liquid with a turpentine odor | |||||||||||||||
| Melting point | −55 ° C | −61 ° C | ||||||||||||||
| boiling point | 155 ° C | 165-166 ° C | ||||||||||||||
| density | 0.86 g cm −3 (15 ° C) | 0.87 g cm −3 (20 ° C) | ||||||||||||||
| Vapor pressure | 5 h Pa (25 ° C) | 2.66 hPa | ||||||||||||||
| solubility | practically insoluble in water | |||||||||||||||
| Refractive index | 1.4653 (20 ° C) | 1.4768 (20 ° C) | ||||||||||||||
| Flash point | 33 ° C | 36 ° C | ||||||||||||||
|
GHS labeling |
|
|
|
|
|
|||||||||||
| H and P phrases | 226-304-315-317 | 226-315-319-335 | 226-304-315-317-410 | see above | see above | |||||||||||
| no EUH phrases | no EUH phrases | no EUH phrases | see above | see above | ||||||||||||
| 280-301 + 310-331 | 310-302 + 352-305 + 351 + 338 | 210-280-301 + 310-331-370 + 378 | see above | see above | ||||||||||||
Occurrence

The α- and β-pinene occur, for example, in myrtle , spruce needles , dill , fennel , coriander and caraway , and in products such as paints, oils and waxes. δ-pinene in rosemary, for example . Oil of turpentine consists of about 60 percent α-pinene.
use
Sandalore , citronellal , camphor , linalool and myrcene can be synthesized from pinene .
biosynthesis
α-pinene and β-pinene are both synthesized from geranyl pyrophosphate by cyclization of linalool pyrophosphate followed by rearrangement of a hydrogen atom.
properties
Pinene are not very volatile, flammable, clear liquids with a turpentine odor, less dense than water, and insoluble in water. Their melting and boiling temperatures and their densities differ only slightly. α-Pinene usually oxidizes to Verbenone , Myrtenol , Pinene Oxide and other products. When exposed to heat, β-pinene can be converted into myrcene . A secondary oxidation product of pinene is the allergenic ascaridol , which is why essential oils containing pinene can cause allergies after just a few days.
α-Pinene belongs to the monoterpenes and is used as a flavoring in the food industry.
α-pinene in higher doses is classified as harmful due to its irritative effects on the eyes, respiratory tract and skin, and possible neurotoxic and nephrotoxic effects. Β-pinene is also irritating. α-pinene may have an anti-inflammatory and at least in vitro anti-microbial effect . In low doses, α-pinene has a bronchospasmolytic effect .
Analytics
For reliable qualitative and quantitative determination, after appropriate sample preparation, the coupling of gas chromatography and mass spectrometry is used. The use of olfactometry is also used for identification and characterization.
Risk assessment
Pin-2 (10) -en was included by the EU in 2014 in accordance with Regulation (EC) No. 1907/2006 (REACH) in the context of substance evaluation in the Community's ongoing action plan ( CoRAP ). The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of Pin-2 (10) -en were concerns about consumer use , high (aggregated) tonnage and widespread use as well as the suspected dangers of sensitizing properties. The re-evaluation took place from 2014 and was carried out by Greece . A final report was then published.
Related links
literature
- Dennis Hobuß: α- and β-Pinene: Versatile Chiral Carbon Scaffolds for Asymmetric Catalysis . WiKu-Wissenschaftsverlag Dr. Stein, Duisburg / Cologne 2007, ISBN 978-3-86553-225-1 .
Individual evidence
- ↑ Z. Szakonyia et al .: Regio- and stereoselective synthesis of the enantiomers of monoterpene-based β-amino acid derivatives. In: Tetrahedron: Asymmetry 18 (20), 2007, pp. 2442-2447, doi: 10.1016 / j.tetasy.2007.09.028 .
- ↑ O. Wallach: On the knowledge of the terpenes and the essential oils about Nopinon. In: Nachr. Ges. Wiss. Goettingen. 1907, p. 232.
- ↑ A. Blumann, O. Zeitschel: About verbena (dehydro-α-pinene) and some of its descendants. In: Chemical Reports . Volume 54, No. 5, 1921, p. 887.
- ↑ Harry Schmidt: On the spatial isomerism in the Pinanzeile, VI. Commun .: cis- and trans-δ-pinene. In: Chemical Reports. 80 (6), 1947, pp. 520-527. doi: 10.1002 / cber.19470800610
- ↑ a b c d e f g h i Entry on alpha-pinene in the GESTIS substance database of the IFA , accessed on December 25, 2019 (JavaScript required)
- ↑ a b c d e f g Entry on beta-pinene in the GESTIS substance database of the IFA , accessed on December 25, 2019 (JavaScript required)
- ^ A b c R. T. O'Connor, LA Goldblatt: Correlation of Ultraviolet and Infrared Spectra of Terpene Hydrocarbons. In: Anal. Chem. 26, 1954, pp. 1726-1737. doi: 10.1021 / ac60095a014 .
- ↑ Data sheet (+) - β-Pinene from Sigma-Aldrich , accessed on August 30, 2018 ( PDF ).
- ^ U. Neuenschwander: Mechanism of the Aerobic Oxidation of α-Pinene . In: ChemSusChem . tape 3 , no. 1 , 2010, p. 75-84 , doi : 10.1002 / cssc.200900228 .
- ↑ ALPHA-PINENE | FEMA. In: www.femaflavor.org. Retrieved July 18, 2016 .
- ↑ Ethan B Russo: Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects . In: British Journal of Pharmacology . tape 163 , no. 7 , August 1, 2011, p. 1344-1364 , doi : 10.1111 / j.1476-5381.2011.01238.x , PMID 21749363 , PMC 3165946 (free full text).
- ↑ Lorenzo Nissen, Alessandro Zatta, Ilaria Stefanini, Silvia Grandi, Barbara Sgorbati: Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.) . In: Fitoterapia . tape 81 , no. 5 , July 1, 2010, p. 413-419 , doi : 10.1016 / j.fitote.2009.11.010 .
- ↑ AA Falk, MT Hagberg, AE Lof, EM Wigaeus-Hjelm, TP Wang: Uptake, distribution and elimination of alpha-pinene in man after exposure by inhalation . In: Scand J Work Environ Health. tape 16 , 1990, pp. 372-378 , PMID 2255878 .
- ↑ I. Bonaccorsi, D. Sciarrone, L. Schipilliti, P. Dugo, L. Mondello, G. Dugo: Multidimensional enantio gas chromtography / mass spectrometry and gas chromatography-combustion-isotopic ratio mass spectrometry for the authenticity assessment of lime essential oils (C. aurantifolia Swingle and C. latifolia Tanaka). In: J Chromatogr A. 1226, Feb 24, 2012, pp. 87-95. PMID 22088669 .
- ↑ H. Sun, T. Zhang, Q. Fan, X. Qi, F. Zhang, W. Fang, J. Jiang, F. Chen, S. Chen: Identification of floral scent in chrysanthemum cultivars and wild relatives by gas chromatography -mass spectrometry. In: Molecules. 20 (4), 25 Mar 2015, pp. 5346-5359. PMID 25816078 .
- ↑ Z. Xiao, B. Fan, Y. Niu, M. Wu, J. Liu, S. Ma: Characterization of odor-active compounds of various Chrysanthemum essential oils by gas chromatography-olfactometry, gas chromatography-mass spectrometry and their correlation with sensory attributes. In: J Chromatogr B Analyt Technol Biomed Life Sci. 1009-1010, Jan 15, 2016, pp. 152-162. PMID 26735711 .
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): (-) - pin-2 (10) -ene , accessed on May 20, 2019.