1,3-pentadiene
| 1,3-pentadienes (penta-1,3-dienes, piperylenes, 1-methyl-1,3-butadienes) | |||||||
| Surname | trans -1,3-pentadiene | cis -1,3-pentadiene | |||||
| other names |
trans -penta-1,3-diene ( E ) -1,3-pentadiene trans -piperylene ( E ) -piperylene |
cis -penta-1,3-diene ( Z ) -1,3-pentadiene cis -piperylene ( Z ) -piperylene |
|||||
| Structural formula | 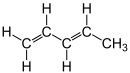 |

|
|||||
| CAS number | 2004-70-8 | 1574-41-0 | |||||
| 504-60-9 (mixture of isomers) | |||||||
| PubChem | 62204 | 643785 | |||||
| Molecular formula | C 5 H 8 | ||||||
| Molar mass | 68.12 g mol −1 | ||||||
| Physical state | liquid | ||||||
| Brief description | colorless liquid | ||||||
| Melting point | −87 ° C | −141 ° C | |||||
| boiling point | 42 ° C | 44 ° C | |||||
| density | 0.683 g cm −3 (25 ° C) | 0.691 g cm −3 (25 ° C) | |||||
| Vapor pressure | 452 hPa (20 ° C) | 453 hPa (20 ° C) | |||||
| Refractive index | 1.430 (20 ° C) | 1.437 (20 ° C) | |||||
| solubility | miscible with ethanol, ether, acetone and benzene | ||||||
|
GHS labeling |
|
|
|||||
| H and P phrases | 225-304-315-319-335 | 225-304 | |||||
| no EUH phrases | no EUH phrases | ||||||
| 210-261-301 + 310-305 + 351 + 338-331 | 210-301 + 310-331 | ||||||
1,3-Pentadiene is an imprecise name for two chemical compounds from the group of dienes that are isomeric to one another (configuration isomers). It is also known as piperylene (a derivative of piperine ).
Another 1,3-pentadiene is the branched isomer 2-methylbutadiene ( isoprene ).
Extraction and presentation
1,3-pentadiene can be prepared by methylation of 1,3-butadiene with dimethyl sulfoxide in the presence of a base such as potassium tert -butoxide are obtained, wherein a mixture of about 80% trans - and 20% cis -1,3-pentadiene produced.
It can also be produced by a Wittig reaction from acetaldehyde , acrolein or crotonaldehyde , both the yield and the cis / trans selectivity varying depending on various factors (e.g. the starting material or the solvent used).
It is also formed in the Hofmann elimination from piperidine , which is converted into 1,4-pentadiene by repeated exhaustive methylation and subsequent elimination of trimethylamine , but which isomerized to 1,3-pentadiene under the reaction conditions.
It is also produced as a by-product of the process of separating crude C 5 materials from pyrolysis gasoline (pygas) - both by-products of the production of ethylene .
properties
1,3-pentadiene is a colorless liquid.
use
1,3-pentadiene can be used to produce other chemical compounds such as. B. 2-methylfuran can be used. It is also used as a monomer in the manufacture of plastics, adhesives, and resins. Piperylene-based products are used particularly in modern adhesives - such as in the production of envelopes, packaging tape and diaper fasteners - as well as in road markings around the world.
Individual evidence
- ↑ a b c d e f g h data sheet trans-1,3-pentadiene, 90% from Sigma-Aldrich , accessed on October 13, 2012 ( PDF ).
- ↑ a b c d e f data sheet cis-1,3-pentadiene, 98% from Sigma-Aldrich , accessed on October 13, 2012 ( PDF ).
- ↑ a b Entry on trans-1,3-pentadienes in the Hazardous Substances Data Bank , accessed October 13, 2012.
- ↑ C. Schotten: Contribution to the knowledge of piperidine. In: Reports of the German Chemical Society 15, 1882, pp. 421-427, doi : 10.1002 / cber.18820150186 .
- ↑ Jerome Thomas Kresse: The investigation of factors influencing the stereochemistry of the Wittig reaction (PDF; 3.9 MB), University of Florida, Diss., 1965.
- ↑ Albert Gossauer: Structure and reactivity of biomolecules . John Wiley & Sons, 2003, ISBN 3-906390-29-2 , pp. 255 ( limited preview in Google Book search).
- ^ A b Shell Chemicals: Piperylene product overview ( Memento from September 18, 2013 in the Internet Archive ).


