1-deoxygalactonojirimycin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Migalastat | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 6 H 13 NO 4 | ||||||||||||
| Brief description |
white crystalline solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 163.17 g mol −1 (free base) | ||||||||||||
| Physical state |
firmly |
||||||||||||
| solubility |
soluble as hydrochloride in water (≥ 1 mg / ml) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
1-Deoxygalactonojirimycin (DGJ), international non-proprietary name Migalastat (trade name: Galafold , manufacturer: Amicus Therapeutics ), is a reversible competitive inhibitor of the enzyme class of galactosidases . Migalastat is the first orally active drug for the treatment of the lysosomal storage disease Fabry disease . The substance acts as a pharmacological chaperone , that is, by binding to the abnormally incorrectly folded enzyme α-galactosidase A , the protein folding process is shifted towards the correct conformation. As a result, the “properly folded” enzyme can perform its function.
description
Chemically, the iminosugar 1-deoxygalactonojirimycin is an analogue of galactose . The similarity to the terminal galactose group of the molecule globotriaosylceramide (Gb3) enables the pharmacological effect in the treatment of Fabry's disease.
Natural occurrence
So far, 1-deoxygalactonojirimycin has not been found in nature. A derivative of DGJ, β-1 C -butyl-DGJ, was isolated in 2000 from the genus Adenophora - a bellflower. In 1988, 1-deoxygalactonojirimycin was obtained for the first time from the fermentation broth of Streptomyces lydicus PA-5726.
Mechanism of action
Migalastat is intended as a pharmacological chaperone to enable the correct folding of mutation variants of the enzyme α-galactosidase A. Due to a gene mutation, Fabry disease patients produce variants of α-galactosidase A in their cells, which, due to their incorrect folding, are separated out by the protein quality control in the endoplasmic reticulum (ER) and fed to the proteasome for degradation. In many cases, these mutant enzyme variants could not only pass protein quality control but also function as an enzyme in the lysosome if correctly folded. Migalastat should intervene in this process by serving as a folding template for α-galactosidase A. This is intended to shift and stabilize the folding dynamics of the protein in the direction of the correct conformation . With the correct tertiary structure , the quality control in the ER is "passed". The stable chaperone-α-galactosidase A complex is transferred through vesicles of the endoplasmic reticulum into the Golgi apparatus and then into the lysosome. There Migalastat is to be replaced by the natural substrate (Gb3). The dissociation of the complex is said to be favored by the high concentration of Gb3 and the low pH value in the lysosome.
The EC 50 values for α-galactosidase A are strongly dependent on the mutation variant present. In in vitro testing of lymphocytes with 49 different missense - mutations that varied EC 50 values ranging from 820 nmol / L to about> 1 mmol / L.
Clinical application
field of use
In May 2016, Migalastat was approved by the EU Commission under the trade name Galafold for the oral long-term treatment of patients aged 16 and over with a confirmed diagnosis of Fabry disease (α-galactosidase A deficiency) who have a mutation that responds to the treatment. Of the more than 800 known mutations in the GLA gene that are susceptible to the effects of migalastat, they were determined in a proprietary in-vitro test. The current approval includes all 269 GLA mutations that were identified based on the test and found to be acceptable. They can be found in about 35 to 50 percent of patients with Fabry disease. It is not recommended for mutations that do not respond, nor is Migalastat intended for simultaneous treatment with enzyme replacement therapy.
The American Food and Drug Administration (FDA) approved Migalastat in August 2018. Approval was granted in the context of an accelerated approval .
Studies
The assessment by the Committee for Medicinal Products for Human Use , which recommended approval in April 2014, is based on clinical data from two Phase III pivot studies in 127 patients and on clinical results from an open-label extension study. The Fabry disease patients had a GLA mutation that responds to migalastat. Both patients who had previously received enzyme replacement therapy with agalsidase alfa / beta (standard therapy ) and were switched to migalastat (ATTRACT study), as well as patients who had not previously received enzyme replacement therapy or who had received enzyme replacement therapy more than six months ago, were examined lag (FACETS study). The change in kidney function of the patients after 18 months of treatment was decisive for assessing the effectiveness . Galafold was found to be as effective as enzyme replacement therapy in stabilizing the patient's kidney function. Galafold also improved cardiac function (lowering the left ventricular mass index ).
Side effects and restrictions on use
The most common side effect was headache, which was seen in approximately 10% of patients who received Galafold .
Restrictions on use may arise if pregnancy is or is planned because insufficient data are available on the effects of Migalastat on the unborn child. It is not known whether Migalastat is excreted in breast milk.
synthesis
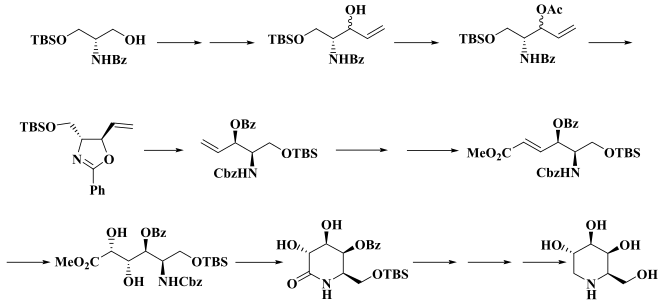
The total synthesis of 1-deoxygalactonojirimycin is relatively complex for such a small molecule. One synthetic route starts with D - N -Benzoylserinol, a derivative of serinol (2-amino-1,3-propanediol), and leads over several stages to a trans - oxazoline (center, left), from which in about 25% yield the 1-deoxygalactonojirimycin can be obtained after eight further steps. Further synthetic routes are described in the literature. An elegant synthesis involves a total of eight steps and has an overall yield of 35 or 43% with over 99% enantiomeric purity .
Trade names
- Galafold (EU)
further reading
- P. Compain, OR Martin (Ed.): Iminosugars: from synthesis to therapeutic applications. Verlag John Wiley and Sons, 2007, ISBN 0-470-03391-6 limited preview in Google Book Search
- K. Suzuki, T. Nakahara, O. Kanie: 3,4-Dihydroxypyrrolidine as glycosidase inhibitor. In: Current Topics in Medicinal Chemistry . Volume 9, Number 1, 2009, pp. 34-57, PMID 19199995 . (Review).
- R. Khanna, R. Soska et al. a .: The pharmacological chaperone 1-deoxygalactonojirimycin reduces tissue globotriaosylceramide levels in a mouse model of Fabry disease. In: Molecular therapy: the journal of the American Society of Gene Therapy. Volume 18, number 1, January 2010, pp. 23-33, doi : 10.1038 / mt.2009.220 . PMID 19773742 . PMC 2839206 (free full text).
- ER Benjamin, JJ Flanagan u. a .: The pharmacological chaperone 1-deoxygalactonojirimycin increases alpha-galactosidase A levels in Fabry patient cell lines. In: Journal of inherited metabolic disease. Volume 32, Number 3, June 2009, pp. 424-440, doi : 10.1007 / s10545-009-1077-0 . PMID 19387866 .
- S. Reissmann: Synthesis and glycosidase inhibitor properties of calystegins. Dissertation, University of Halle-Wittenberg, 2006
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Migalastat
Individual evidence
- ↑ a b c d data sheet Deoxygalactonojirimycin hydrochloride from Sigma-Aldrich , accessed on February 4, 2018 ( PDF ).
- ↑ K. Ikeda, M. Takahashi et al. a .: Homonojirimycin analogues and their glucosides from Lobelia sessilifolia and Adenophora spp. (Campanulaceae). In: Carbohydrate Research . Volume 323, Numbers 1-4, Jan 2000, pp. 73-80, PMID 10782288 .
- ^ Y. Miyake, M. Ebata: The structures of a β-galactosidase inhibitor, galactostatin, and its derivatives. In: Agric Biol Chem. Vol. 52, 1988, pp. 661-666.
- ↑ N. Asano: Naturally occuring iminosugars and related alkaloids: structure, activity and applications. In: P. Compain, OR Martin (Ed.): Iminosugars: from synthesis to therapeutic applications. Verlag John Wiley and Sons, 2007, ISBN 0-470-03391-6 , p. 17. Restricted preview in the Google book search
- ^ S. Ishii, HH Chang et al. a .: Preclinical efficacy and safety of 1-deoxygalactonojirimycin in mice for Fabry disease. In: Journal of pharmacology and experimental therapeutics . Volume 328, Number 3, March 2009, pp. 723-731, doi : 10.1124 / jpet.108.149054 . PMID 19106170 .
- ↑ R. Hamanaka, T. Shinohara et al. a .: Rescue of mutant alpha-galactosidase A in the endoplasmic reticulum by 1-deoxygalactonojirimycin leads to trafficking to lysosomes. In: Biochimica et biophysica acta . Volume 1782, number 6, June 2008, pp. 408-413, doi : 10.1016 / j.bbadis.2008.03.001 . PMID 18381081 .
- ↑ S. Biastoff, B. Dräger: Calystegines. In: GA Cordell (Ed.): The Alkaloids: Chemistry and Biology. P. 91. Limited preview in Google Book search
- ↑ GH Yam, N. Bosshard et al. a .: Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. In: American Journal of Physiology-Cell Physiology . 2006; 290: p. C1076-C1082, doi : 10.1152 / ajpcell.00426.2005 . PMID 16531566 .
- ↑ ER Benjamin, JJ Flanagan u. a .: The pharmacological chaperone 1-deoxygalactonojirimycin increases alpha-galactosidase A levels in Fabry patient cell lines. In: Journal of inherited metabolic disease. Volume 32, Number 3, June 2009, pp. 424-440, doi : 10.1007 / s10545-009-1077-0 . PMID 19387866 .
- ↑ Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: 1-Deoxygalactonojirimycin
- ↑ FDA approves new treatment for a rare genetic disorder, Fabry disease , PM FDA of August 10, 2018, accessed on September 14, 2018
- ↑ Galafold . European Medicines Agency . April 1, 2016.
- ↑ SJ Pyun, KY Lee et al. a .: Synthesis Of (+) - 1-Deoxygalactonojirimycin. In: Heterocycles . Volume 62, 2004, pp. 333-341.
- ^ AB Hughes, AJ Rudge: Deoxynojirimycin: synthesis and biological activity. In: Natural Product Reports . Volume 11, Number 2, April 1994, pp. 135-162, PMID 15209127 . (Review).
- ↑ OK Karjalainen, AMP Koskinen: Rapid and practical synthesis of (-) - 1-deoxyaltronojirimycin. In: Org Biomol Chem. Volume 9, 2011, pp. 1231-1236. doi : 10.1039 / C0OB00747A
- ↑ OK Karjalainen, M. Passiniemi, AM Koskinen: Short and straightforward synthesis of (-) - 1-deoxygalactonojirimycin. In: Organic letters. Volume 12, Number 6, March 2010, pp. 1145-1147, doi : 10.1021 / ol100037c . PMID 20170191 .