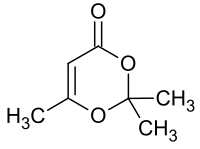
2,2,6-trimethyl-4 H -1,3-dioxin-4-one
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,2,6-trimethyl-4 H -1,3-dioxin-4-one | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 10 O 3 | ||||||||||||||||||
| Brief description |
clear, brown liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 142.16 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.0879 g cm −3 (20 ° C ) |
||||||||||||||||||
| Melting point |
12-13 ° C |
||||||||||||||||||
| boiling point | |||||||||||||||||||
| solubility |
practically insoluble in water, miscible with most organic solvents |
||||||||||||||||||
| Refractive index |
1.4678 (25 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
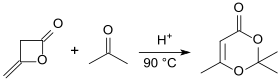
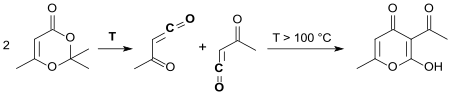
2,2,6-Trimethyl-4 H -1,3-dioxin-4-one is a six-membered heterocycle with two oxygen atoms in the 1,3-position with the basic structure of 1,3-dioxane . The compound is the 1: 1 adduct of diketene and acetone and is used in chemical syntheses as the equivalent of the poisonous, foul-smelling and tear-irritating diketene. Diketene-acetone adduct (DAA) breaks down into acetyl ketene when heated, which can form a large number of acetoacetic acid derivatives with nucleophiles .
Occurrence and representation
The synthesis, structure and properties of 2,2,6-trimethyl-4 H -1,3-dioxin-4-one were first clearly and comprehensively described in 1952 by MF Carroll and Alfred Bader .
Diketene reacts with acetone at 90 ° C in the presence of catalytic amounts of p -toluenesulfonic acid in 91% yield to form DAA. The reaction can also be catalyzed by quaternary ammonium compounds .
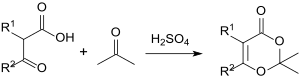
Substituted 2,2-dimethyl-1,3-dioxin-4-ones are formed in the reaction of β- keto acids with acetone.
properties
2,2,6-Trimethyl-4 H -1,3-dioxin-4-one is an inflammable, yellow-brown liquid with a pleasant odor. The compound is practically insoluble in water and mixes with most organic solvents. Technical DAA usually still contains small amounts (usually up to 6%) of acetone. Because of the decomposition at higher temperatures, the purification of the diketene-acetone adduct must be carried out by fractional distillation at <80 ° C.
Applications
Reactions to DAA
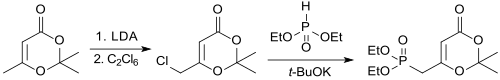
With chlorine in dichloromethane , the diketene-acetone adduct is converted into the chloromethyl compound at -50 ° C. in the 5-position in quantitative yield.
The 5-chloromethyl derivative reacts smoothly with sodium methoxide (94% yield) to give methyl α-chloroacetoacetate.
Chlorine can be activated at the methyl group in 6-position by reaction with lithium diisopropylamide LDA in THF was treated with hexachloroethane be introduced
The corresponding phosphonoalkyl compound is obtained from the chloromethyl compound with the potassium salt of diethyl phosphite , which in the thermolysis in the presence of z. B. forms amino acid esters β-keto acid amides.
These β-keto acid amides can easily be cyclized with sodium methoxide to give unsaturated tetramic acids .
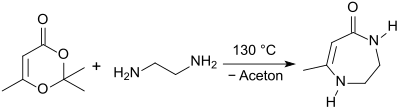
When 2,2,6-trimethyl-4 H -1,3-dioxin-4-one is reacted with α, ω- diamines by simply heating to 130 ° C for 5 minutes under microwave irradiation , the difficult to access "middle rings" are with 7-10 ring members obtained in quantitative yield.
Reactions with acetyl ketene
By thermal retro-Diels-Alder reaction at temperatures above 100 ° C, the reactive acetyl ketene is formed from 2,2,6-trimethyl-4 H -1,3-dioxin-4-one, which at temperatures> 130 ° C in xylene dimerized practically quantitatively to dehydroacetic acid in a [4 + 2] cycloaddition .
Dehydroacetic acid was obtained as early as 1953 in 51% yield when diketene-acetone adduct was heated in toluene in the presence of the weak base calcium acetate , without acetyl ketene being recognized as a reactive intermediate.
Acetylketene reacts in situ with nucleophiles, such as B. alcohols, phenols, thiols and amines in high yields to derivatives of acetoacetic acid.
Side reactions in the reaction with alcohols and amines are suppressed if the reaction with 2,2,6-trimethyl-4 H -1,3-dioxin-4-one in tetrahydrofuran is carried out under reflux in the presence of stoichiometric amounts of sodium acetate .
Practically quantitative conversions can also be achieved with sterically hindered nucleophiles.
In a hetero-Diels-Alder reaction ([4 + 2] cycloaddition), six-membered heterocycles with a carbon-carbon double bond are formed with dienophiles with the structure X = Y.
The Biginelli reaction as a multicomponent reaction in the one- pot process gives the N-phenylacetoacetanilide (94% yield) even in boiling water as a solvent with 2,2,6-trimethyl-4 H -1,3-dioxin-4-one and aniline Ethanol reacts with urea and the aldehyde piperonal with 86% yield to form the corresponding dihydropyrimidine derivative .
3,4-Dihydropyrimidin-2 (1 H ) -ones (DHPMs) are based on a large number of pharmacologically active substances.
Individual evidence
- ↑ a b c data sheet 2,2,6-trimethyl-4H-1,3-dioxin-4-ones from Sigma-Aldrich , accessed on December 20, 2019 ( PDF ).
- ↑ a b c d e W.LF Armarego, CLL Chai: Purification of Laboratory Chemicals, 7th Edition . Elsevier Inc., Amsterdam 2013, ISBN 978-0-12-382161-4 , pp. 493 .
- ↑ a b R.J. Clemens: 2,2,6-trimethyl-4H-1,3-dioxin-4-ones . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2001, doi : 10.1002 / 047084289X.rt272 .
- ↑ a b M.F. Carroll, AR Bader: The reaction of diketene with ketones . In: J. Am. Chem. Soc. tape 74 , no. 24 , 1952, pp. 6305 , doi : 10.1021 / ja01144a530 .
- ↑ a b c M.F. Carroll, AR Bader: The reactions of diketene with ketones . In: J. Am. Chem. Soc. tape 75 , no. 21 , 1953, pp. 5400-5402 , doi : 10.1021 / ja01117a076 .
- ↑ EV Dehmlow, AR Shamout: Onium salt-catalyzed reactions of carbonyl compounds with diketene . In: Justus Liebigs Ann. Chem. Band 1982 , no. 9 , 1952, pp. 1753-1755 , doi : 10.1002 / jlac.198219820917 .
- ↑ M. Sato, H. Ogasawara, K. Oi, T. Kato: Synthesis of 1,3-Dioxin-4-one derivatives . In: Chem. Pharm. Bull. Volume 31 , no. 6 , 1983, pp. 1896-1901 , doi : 10.1248 / cpb.31.1896 .
- ↑ Patent US4633013 : Preparation of α-haloacetoacetic acid esters. Applied on June 11, 1984 , published December 30, 1986 , applicant: Eastman Kodak Co., inventor: RJ Clemens.
- ↑ RK Boeckman, Jr., RB Perni, JE Macdonald, AJ Thomas: 6-Diethylphosphonomethyl-2,2-dimethyl-1,3-dioxen-4-one In: Organic Syntheses . 66, 1988, p. 194, doi : 10.15227 / orgsyn.066.00194 ; Coll. Vol. 8, 1993, p. 192 ( PDF ).
- ↑ CS Morrison, JB Lampe, TC Kolodziejczyk, RJ Cavazos, RA Petros: Rapid, quantitative, solvent-free synthesis of medium-ring diaza heterocycles from diketene-acetone adduct and diamines . In: Tetrahedron Lett. tape 55 , no. 48 , 2014, p. 6547-6549 , doi : 10.1016 / j.tetlet.2014.10.007 .
- ↑ Patent US4496747 : Process for the preparation of dehydracetic acid. Applied on April 21, 1983 , published January 29, 1985 , applicant: Eastman Kodak Co., inventor: RJ Clemens.
- ↑ RJ Clemens, JA Hyatt: Acetoacetylation with 2,2,6-Trimethyl-4 H -1,3-dioxin-4-one: A convenient alternative to diketene . In: J. Org. Chem. Band 50 , no. 14 , 1985, pp. 2431-2435 , doi : 10.1021 / jo000214a006 .
- ↑ V. Sridharan, M. Ruiz, JC Menéndez: Mild and high-yielding synthesis of β-keto esters and β-ketoamides . In: Synthesis . tape 2010 , no. 6 , 2010, p. 1053-1057 , doi : 10.1055 / s-0029-1217135 .
- ↑ G. Jäger, J. Wenzelburger: Acylketene II1), heterocycle syntheses by cycloadditions with acylketenes . In: Justus Liebigs Ann. Chem. Band 1976 , no. 9 , 1976, p. 1689-1712 , doi : 10.1002 / jlac.197619760917 .
- ↑ FHS Gama, ROMA de Souza, SJ Garden: An efficient green protocol for the preparation of acetoacetamides and application of the methodology to a one-pot synthesis of Biginelli dihydropyrimidines. Expansion of dihydropyrimidine topological chemical space . In: RSC Adv. Band 5 , no. 87 , 2015, p. 70915-70928 , doi : 10.1039 / C5RA14355A .