2,5 furandicarboxylic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,5 furandicarboxylic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 4 O 5 | ||||||||||||||||||
| Brief description |
White dust |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 156.09 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.73 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
> 320 ° C |
||||||||||||||||||
| boiling point |
419.2 ° C |
||||||||||||||||||
| pK s value |
2.60 |
||||||||||||||||||
| solubility |
soluble in DMSO , dimethylformamide and acetic acid , ~ 1 g / l 18 ° C water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2,5-furandicarboxylic acid (FDCA) is a dicarboxylic acid obtained by dehydration of hexoses and oxidation of the resulting hydroxymethyl furfural (5-HMF) can be generated. It serves as a starting material for the production of biopolymers .
history
2,5-furandicarboxylic acid was first synthesized in 1876 as dehydroglucic acid by Rudolph Fittig and Robert Heinzelmann by reacting mucic acid with fuming hydrobromic acid.
The US Department of Energy identified the compound as one of the twelve most important platform chemicals (biobased building block chemicals) of the “green, renewable chemistry” of the future.
Synthesis and representation
There are different routes for displaying the FDCA:
Oxidation of 2,5-disubstituted furans
Starting from 2,5-disubstituted furans, 2,5-furandicabonic acid can be obtained in almost quantitative yield with various inorganic oxidants or by noble metal-catalyzed oxidation . 5-Hydroxymethylfurfural, which in turn can be obtained from fructose, is particularly suitable as the starting material for the oxidation. The 2,5-furandicarboxylic acid can also be obtained from 5-hydroxymethylfurfural by a biocatalytic route. The electrochemical oxidation of HMF to 2,5 ‐ furandicarboxylic acid takes place in acidic media with the help of a manganese oxide anode (MnOx) with a yield of approx. 50%.
Synthesis from furfural
Catalytic oxidation first converts furfural with nitric acid to furan-2-carboxylic acid and then converts it into the methyl ester. This is then substituted by chloromethylation at position 5 to give the 5- (chloromethyl) -furan-2-carboxylic acid methyl ester. Reaction with nitric acid gives dimethylfuran-2,5-dioate, from which FDCA is obtained after alkaline hydrolysis with 50% yield.
Dehydration of mucic acid
A single-stage, acid-catalyzed dehydration of mucic acid, also known as mucic acid or galactar acid, in butanol under strongly acidic reaction conditions (e.g. sulfurous acid , p -toluenesulfonic acid and heteropolyacids) makes the dibutyl ester of 2,5-furandicarboxylic acid accessible.
Properties and uses
Since the furandicarboxylic acid has two carboxyl groups, typical reactions for carboxylic acids, such as e.g. B. the formation of carboxylic acid dihalides, carboxylic acid esters and carboxylic acid amides possible. Polycondensation reactions can be carried out with diols and diamines . A large number of polyesters , polyamides or polyurethanes are available starting from the FDCA monomer . Analogous to the polymerization of terephthalic acid , the furan dicarboxylic acid with ethylene glycol to Polyethylenfuranoat (PEF) or butyl diglycol to Polybutylenfuranoat (PBF) are polymerized.
FDCA can be used directly in many applications or as a platform chemical in the synthesis of biopolymers.
Direct use
- Plasticizers: FDCA esters can be used as a substitute for phthalates as plasticizers in polyvinyl chloride , e.g. B. in PVC cables.
- Fire extinguishing foam: FDCA and most polycarboxylic acids are components of fire extinguishing foams.
- Pharmaceuticals: In pharmacy, the ethyl ester of FDCA has been shown to cause an anesthetic effect similar to cocaine. Screening studies on some FDCA derivatives showed important antibacterial properties. To prepare artificial veins for transplantation, a dilute solution of FDCA in tetrahydrofuran is used.
- Detergents: FDCA is used as a component of optical brighteners in detergents.
Use as a platform chemical
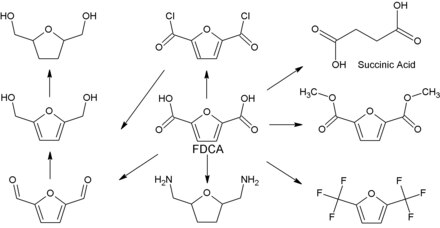
Various derivatizations of 2,5-furandicarboxylic acid, for example to 2,5-bis (hydroxymethyl) furan , 2,5-bis (hydroxmethyl) tetrahydrofuran or 2,5-bis (aminomethyl) tetrahydrofuran, result in a variety of other possible uses:
Manufacturer
Alfa Aesar GmbH & Co KG; Asta Tech Inc .; AVALON Industries AG; Avantium Holding BV; Baxter; Bristol-Myers Squibb; Chemsky (Shanghai) International Company Ltd; Corbion NV; Cryolife; CSL Behring; Davol; Ethicon; Genzymes; Medtronic; Novamont SpA; Pfizer; Synvina .; Takeda; Toronto Research Chemicals Inc; V&V Pharma Industries
literature
- Muhammad Sajid, Xuebing Zhao, Dehua Liu: Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): recent progress focusing on the chemical-catalytic routes (Review) . In: Green Chemistry . Advance Article, October 2018. doi : 10.1039 / C8GC02680G .
- Guangquan Chen, Nico M. van Straalen, Dick Roelofs: The ecotoxicogenomic assessment of soil toxicity associated with the production chain of 2,5-furandicarboxylic acid (FDCA), a candidate bio-based green chemical building block . In: Green Chemistry . 18, No. 16, May 2016. doi : 10.1039 / C6GC00430J .
- WATSON INTERNATIONAL LTD: 2,5-Furandicarboxylic acid, (SAFETY DATA SHEET) In: Version 5.0 . WATSON INTERNATIONAL LTD. Pp. 1-7. June 3, 2017. Retrieved November 22, 2018.
Web links
Individual evidence
- ↑ a b Entry on 2,5-furandicarboxylic acid in the ChemSpider database of the Royal Society of Chemistry , accessed on November 22, 2018.
- ↑ Registration dossier on Furan-2,5-dicarboxylic acid ( Density section ) at the European Chemicals Agency (ECHA), accessed on February 13, 2019.
- ^ A b J. Buckingham: Dictionary of Organic Compounds . CRC Press, 1996, ISBN 978-0-412-54090-5 , pp. 3264 ( limited preview in Google Book Search).
- ↑ Yongzhao Zhang, Zhang, Xia Guo, Ping Tang, Jian Xu: Solubility of 2,5-Furandicarboxylic Acid in Eight Pure Solvents and Two Binary Solvent Systems at 313.15-363.15 K . In: Journal of chemical & engineering data . 63, No. 5, 2018, pp. 1316–1324. doi : 10.1021 / acs.jced.7b00927 .
- ↑ a b Registration dossier on Furan-2,5-dicarboxylic acid ( GHS section ) at the European Chemicals Agency (ECHA), accessed on December 1, 2018.
- ↑ clearsynth: 2,5-Furandicarboxylic Acid ( Memento from January 2, 2014 in the Internet Archive )
- ↑ Rudolph Fittig: About new derivatives of mucic acid . In: Reports of the German Chemical Society . 9, No. 2, July 1876, pp. 1189-1199. doi : 10.1002 / cber.18760090250 .
- ↑ Robert Heinzelmann: About some new derivatives of mucic acid (dissertation) . University of Strasbourg 1876 ( limited preview in Google book search).
- ↑ E. de Jong, MA Dam, L. Sipos, G.-JM Gruter: Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters . In: ACS Symposium Series . tape 1105 . American Chemical Society, Washington, DC 2012, ISBN 978-0-8412-2767-5 , pp. 1–13 , doi : 10.1021 / bk-2012-1105.ch001 .
- ^ A b Joseph J. Bozell, Gene R. Petersen: Technology development for the production of biobased products from biorefinery carbohydrates — the US Department of Energy's "Top 10" revisited . In: Green Chemistry . 12, 2010, pp. 539-554. doi : 10.1039 / B922014C .
- ↑ a b c d FDCA (2,5-furandicarboxylic acid) biorefineries BioRefineries Blog, June 15, 2017.
- ↑ a b c d Jaroslaw Lewkowski: Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives . In: ARKAT USA (Ed.): ARKIVOC . tape 2001 , 2001, p. 17-54 , doi : 10.3998 / ark.5550190.0002.102 .
- ↑ P. Verdeguer, N. Merat, A. Gaset: Oxydation catalytique du HMF en acid 2,5-furane dicarboxylique . In: Journal of Molecular Catalysis . 85, 1993, pp. 327-344. doi : 10.1016 / 0304-5102 (93) 80059-4 .
- ↑ Sara E. Davisa, Levi R. Houkb, Erin C. Tamargoa, K. Abhaya, Robert Datyeb, J. Davisa: Oxidation of 5-hydroxymethylfurfural next term over supported Pt, Pd and Au catalysts . In: Catalysis Today . 160, No. 1, February 2, 2011, pp. 55-60. doi : 10.1016 / j.cattod.2010.06.004 .
- ↑ Martin Kröger, Ulf Prüße, Klaus-Dieter Vorlop: A new approach for the production of 2,5-furandicarboxylic acid by in situ oxidation of 5-hydroxymethylfurfural starting from fructose . In: Topics in Catalysis . tape 3 , no. 13 , 2000, pp. 237-242 , doi : 10.1023 / A: 1009017929727 .
- ↑ Ali Hussain Motagamwala, Wangyun Won1, Canan Sener, David Martin Alonso1, Christos T. Maravelias1, James A: Toward biomass-derived renewable plastics: Production of 2,5-furandicarboxylic acid from fructose . In: Science Advances . 4, No. 1, January 2018, p. 9722. doi : 10.1126 / sciadv.aap9722 .
- ↑ Haibo Yuan, Jianghua Lia, Hyun-dong Shinc, Guocheng Du, Jian Chen, Zhongping Shi, Long Liuab: Improved production of 2,5-furandicarboxylic acid by overexpression of 5-hydroxymethylfurfural oxidase and 5-hydroxymethylfurfural / furfural oxidoreductase in Raoultella ornithinol BF60 . In: Bioresource Technology . 247, January 2017, pp. 1184–1188. doi : 10.1016 / j.biortech.2017.08.166 .
- ↑ Gazi Sakir Hossain, Haibo Yuan, Jianghua Li, Hyun-dong Shin, Miao Wang, a Guocheng Du, Jian Chen, Long Liu: Metabolic Engineering of Raoultella ornithinolytica BF60 for Production of 2,5-Furandicarboxylic Acid from 5-Hydroxymethylfurfural . In: Applied and Environmental Microbiology . 83, No. 1, January 2017, pp. 2312-2316. doi : 10.1128 / AEM.02312-16 . PMID 27795308 . PMC 5165124 (free full text).
- ^ Wageningen University & Research: FDCA production from renewable biomass . December 31, 2014. Accessed November 22, 2018.
- ^ SR Kubota, Ks Choi: Electrochemical Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid (FDCA) in Acidic Media Enabling Spontaneous FDCA Separation . In: Chemsuschem . 11, July 2018, pp. 2138-2145. doi : 10.1002 / cssc.201800532 .
- ↑ Fabrizio Cavani, Stefania Albonetti, Francesco Basile, Alessandro Gandini: 2,5-Furandicarboxylic Acid Synthesis and Use . In: Chemicals and Fuels from Bio-Based Building Blocks . tape 1 . WILEY-VCH, 2016, ISBN 978-3-527-33897-9 , Chapter 8, pp. 191-213 .
- ↑ Yoichi Taguchi, Akihiro Oishi, Hiroshi Iida: One-step Synthesis of Dibutyl Furandicarboxylates from Galactaric Acid. In: Chemistry Letters. 37, 2008, p. 50, doi : 10.1246 / cl.2008.50 .
- ^ A b A. Aden, J. Bozell, J. Holladay, J. White, Amy Manheim: Top Value Added Chemicals from Biomass. Volume I - Results of Screening for Potential Candidates from Sugars and Synthesis Gas. Produced by the Staff at Pacific Northwest National Laboratory (PNNL); National Renewable Energy Laboratory (NREL), Office of Biomass Program (EERE), August 2004
- ↑ a b Oliver Türk: Material use of renewable raw materials, basics - materials - applications , Springer Vieweg 2014, chapter 7.2.2, pages 392–393, ISBN 978-3-8348-1763-1 , doi : 10.1007 / 978-3-8348 -2199-7
- ↑ Brandon Evans, 2,5-Furandicarboxylic Acid (FDCA) Market - 2020 Industry Trends, Size, Growth, Share, Emerging Technologies, Share, Competitiveness, Regional and Global Industry Forecast to 2026 ; Munchen Zeitung February 19, 2020
- ↑ Amber Gerald: 2,5-Furandicarboxylic Acid (FDCA) Market Size, Share, Global Industry Growth 2020, Trends, Revenues, Requirements, Key Players, Emerging Technologies, and Industry Potential to 2026 , Schwäbisch Expert February 12, 2020

![Oxidation routes from HMF to FDCA [12]](https://upload.wikimedia.org/wikipedia/commons/thumb/a/a4/Oxidation_of_HMF.svg/550px-Oxidation_of_HMF.svg.png)