2-ethylaniline
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
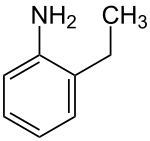
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-ethylaniline | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 11 N | ||||||||||||||||||
| Brief description |
yellow-orange liquid with an aromatic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 121.18 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.98 g cm −3 |
||||||||||||||||||
| Melting point |
−46 ° C |
||||||||||||||||||
| boiling point |
210 ° C |
||||||||||||||||||
| Vapor pressure |
<0.1 hPa (50 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.559 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
2-Ethylaniline is a chemical compound from the group of aniline derivatives .
Extraction and presentation
2-Ethylaniline can be obtained by ethylating aniline using ethene and aluminum trichloride / aluminum as catalysts at a pressure of approx. 200 bar in an autoclave .
properties
2-Ethylaniline is a flammable, hardly flammable, yellow-orange liquid with an aromatic odor, which is practically insoluble in water. It turns brown in air.
use
2-Ethylaniline is used as an intermediate in the manufacture of drugs (e.g. Etodolac ), pesticides, and other chemical compounds.
safety instructions
The vapors of 2-ethylaniline can form an explosive mixture with air ( flash point 92 ° C, ignition temperature approx. 515 ° C).
Individual evidence
- ↑ a b c d e f g h i j k Entry on 2-ethylaniline in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ^ A b Robert A. Lewis: Hawley's Condensed Chemical Dictionary . John Wiley & Sons, 2016, ISBN 978-1-119-26784-3 , pp. 575 ( limited preview in Google Book search).
- ↑ a b Data sheet 2-Ethylaniline, 98% from Sigma-Aldrich , accessed on December 2, 2016 ( PDF ).
- ^ Wilhelm Foerst: Newer Methods of Preparative Organic Chemistry . Elsevier, 1963, ISBN 978-0-323-15042-2 , pp. 230 ( limited preview in Google Book search).
- ↑ Richard P. Pohanish: Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens . William Andrew, 2011, ISBN 978-1-4377-7870-0 , pp. 1198 ( limited preview in Google Book search).

