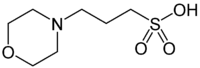
3- ( N -morpholino) propanesulfonic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 3- ( N -morpholino) propanesulfonic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 7 H 15 NO 4 S | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 209.3 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
283.5-284.5 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
3- ( N -Morpholino) propanesulfonic acid (MOPS) is a biochemical buffer substance from the group of morpholines .
properties
MOPS is a zwitterionic Good buffer with a pK a value of 7.2 (with ΔpK a / ° C = −0.013 to −0.015), which is why it is suitable for buffering at neutral pH values . Like all Good buffers, MOPS was developed to minimize interactions with proteins , high solubility , no diffusion through biomembrane , a buffer range between pH 6 and 8, low toxicity , low UV absorption , and independence of the buffer effect from other factors , be inexpensive to manufacture and have metabolic and chemical stability. MOPS has little tendency to complex metal ions, as do 2- ( N -morpholino) ethanesulfonic acid (MES) and 2,2 '- (1,4-piperazinediyl) diethanesulfonic acid (PIPES). MES is a buffer substance structurally related to MOPS, which in turn is structurally related to HEPES . MOPS can increase the thermostability of bovine serum albumin in solutions. Peroxynitrite releases nitric oxide after reacting with MOPS . MOPS partially degrades when autoclaved in the presence of glucose .
application
MOPS is used in protein purification , e.g. B. in chromatography , agarose gel electrophoresis and polyacrylamide gel electrophoresis such as the cationic PAGE and SDS-PAGE . In the cell culture of mammalian cells , MOPS is used as a non-toxic buffer substance at a concentration below 20 mM. In bacterial cell culture, the metabolic acidification of the cell culture medium is slowed down by adding MOPS. MOPS can be added to the PCR buffer in the course of a PCR optimization .
literature
- LN Roy, RN Roy, KA Allen, CJ Mehrhoff, IB Henson, JM Stegner: Buffer standards for the physiological pH of the zwitterionic compound of 3- (N-morpholino) propanesulfonic acid (MOPS) from T = (278.15 to 328.15) K . In: The Journal of chemical thermodynamics. Volume 47, April 2012, pp. 21-27, doi: 10.1016 / j.jct.2011.09.010 . PMID 22247568 . PMC 3254115 (free full text).
Individual evidence
- ↑ Entry on MORPHOLINOPROPANE SULFONIC ACID in the CosIng database of the EU Commission, accessed on July 3, 2020.
- ↑ a b c d e f data sheet MOPS, BioPerformance Certified, cell culture tested, ≥99.5% (titration) from Sigma-Aldrich , accessed on December 19, 2014 ( PDF ).
- ^ R. Goldberg, Kishore, N .; Lennen, R .: Thermodynamic Quantities for the Ionization Reactions of Buffers . In: J. Phys. Chem. Ref. Data . 31, No. 2, 2002, pp. 231-370. doi : 10.1063 / 1.1416902 .
- ^ NE Good, GD Winget, W. Winter, TN Connolly, S. Izawa, RM Singh: Hydrogen ion buffers for biological research. In: Biochemistry . Volume 5, Number 2, February 1966, pp. 467-477, PMID 5942950 .
- ↑ C. Montigny, P. Champeil: Use of metallochromic dyes and potentiometric pH-meter titration to detect binding of divalent cations to "Good's" buffers: 4-morpholinepropanesulfonic acid (Mops) does not bind Mg2 +. In: Analytical Biochemistry . Volume 366, number 1, July 2007, pp. 96-98, doi: 10.1016 / j.ab.2007.02.025 . PMID 17407760 .
- ^ BS Gupta, M. Taha, MJ Lee: Buffers more than buffering agent: introducing a new class of stabilizers for the protein BSA. In: Physical chemistry chemical physics: PCCP. Volume 17, Number 2, January 2015, pp. 1114–1133, doi: 10.1039 / c4cp04663c . PMID 25415385 .
- ^ K. Schmidt, S. Pfeiffer, B. Mayer: Reaction of peroxynitrite with HEPES or MOPS results in the formation of nitric oxide donors. In: Free Radical Biology and Medicine . Volume 24, Number 5, March 1998, pp. 859-862, PMID 9586817 .
- ^ The Merck Index, 12th Edition, Entry # 6346.
- ↑ CR Narahari, JC Strong, DD Frey: Displacement chromatography of proteins using a self-sharpening pH front formed by adsorbed buffering species as the displacer. In: Journal of Chromatography A . Volume 825, Number 2, November 1998, pp. 115-126, PMID 9842719 .
- ↑ J. Sambrook , T. Maniatis , DW Russel: Molecular cloning: a laboratory manual. 3rd edition (2001), Cold Spring Harbor Laboratory Press. ISBN 0-87969-577-3 .
- ↑ JM Thomas, ME Hodes: A new discontinuous buffer system for the electrophoresis of cationic proteins at near-neutral pH. In: Analytical biochemistry. Volume 118, Number 1, November 1981, pp. 194-196, PMID 6278979 .
- ↑ Harry Eagle : Buffer combinations for mammalian cell culture. In: Science . Volume 174, Number 4008, October 1971, pp. 500-503, PMID 5110427 .
- ↑ GA Somkuti, SE Gilbreth: Influence of organic buffers on bacteriocin production by Streptococcus thermophilus ST110. In: Current microbiology. Volume 55, Number 2, August 2007, pp. 173-177, doi: 10.1007 / s00284-007-0179-x . PMID 17632754 .
- ^ A. Ahmad, J. Ghasemi: New buffers to improve the quantitative real-time polymerase chain reaction. In: Bioscience, Biotechnology, and Biochemistry . Volume 71, number 8, August 2007, pp. 1970–1978, doi: 10.1271 / bbb.70164 . PMID 17690445 .
