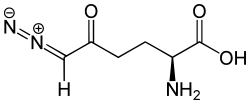
6-diazo-5-oxo- L- norleucine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 6-diazo-5-oxo- L- norleucine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 9 N 3 O 3 . | ||||||||||||||||||
| Brief description |
yellowish solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 171.15 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
6-Diazo-5-oxo- L -norleucine ( L-DON ) is a non-proteinogenic amino acid that acts as an analogue of glutamine . It belongs to the diazo compounds . L-DON was isolated from bacteria of the genus Streptomyces in 1956 and was first described and proposed as a possible chemotherapeutic agent for the treatment of cancer. An anti-tumor effect has been confirmed in various animal experiments. In clinical trials, DON has been tested as a chemotherapy drug against cancer, but has never been approved. The last clinical study on DON to date was published in 2008, here it was administered in combination with a glutaminase .
chemistry
DON is a yellowish powder that is readily soluble in water, but it also dissolves in aqueous solutions of methanol, acetone or ethanol, but only poorly in absolute alcohols. Solutions of at least 50 µM DON in 0.9% saline solution appear slightly yellowish. Yellowish green needles form in crystalline form. The specific rotation is [α] D 26 = + 21 ° (c = 5.4% in H 2 O). In phosphate buffer at pH 7, the ultraviolet absorption maxima are at 274 nm (E1% 1cm. 683) and 244 nm (E1% 1cm 376).
biochemistry
DON is widely used as an inhibitor of various glutamine-converting enzymes. Due to its similarity to glutamine, it finds access to the catalytic centers of these enzymes and blocks it through covalent bonds or alkylation . The following table lists some enzymes that are inhibited by DON.
|
Mechanism of action
As a cell poison, DON has an inhibitory effect on many key enzymes in nucleotide synthesis. When DON was added, apoptosis (programmed cell death) was triggered in various cancer cell lines . Various reasons have been investigated, but the exact mechanism has not been definitively established. On the one hand, the inner mitochondrial membrane was damaged by the administration of DON, and single-strand breaks in the DNA were also detected.
pharmacology
DON is not approved as a drug, but is being tested in clinical trials in combination with a glutaminase for use as a chemotherapy agent against cancer.
Individual evidence
- ↑ a b c Dion HW et al .: 6-diazo-5-oxo-L-norleucine, A new tumor inhibitory substance. II: Isolation and Characterization , in: Antibiotics and Chemotherapy, Vol 78, 1954, 3075-3077.
- ↑ a b c Data sheet 6-Diazo-5-oxo-L-norleucine from Sigma-Aldrich , accessed on March 18, 2011 ( PDF ).
- ↑ Cooney DA, Jayaram HN, Milman HA, et al. : DON, CONV and DONV-III. Pharmacologic and toxicologic studies . In: Biochem. Pharmacol. . 25, No. 16, August 1976, pp. 1859-70. doi : 10.1016 / 0006-2952 (76) 90190-8 . PMID 9092 .
- ↑ Yoshioka K et al. Glutamine antagonist with diet deficient in glutamine and aspartate reduce tumor growth , in: Tokushima J Exp Med. 1992 Jun; 39 (1-2): 69-76; PMID 1412455 .
- ↑ a b Mueller C et al. A phase IIa study of PEGylated glutaminase (PEG-PGA) plus 6-diazo-5-oxo-L-norleucine (DON) in patients with advanced refractory solid tumors ( Memento of the original from September 27, 2011 in the Internet Archive ) Info: Der Archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , in: J Clin Oncol 26: 2008 (May 20 suppl; abstr 2533).
- ↑ DeWald HA and Moore AM: 6-Diazo-5-oxo-L-norleucine, a new tumor-inhibitory substance. Preparation of L (D and L) forms, in Am. Chem. Soc. Meeting, Dallas, 1956, p. 13.
- ↑ a b c d e f g h i Pinkus LM et al. Glutamine binding sites , in: Methods Enzymol, 1977, Vol. 46, 414-427.
- ↑ Ortlund E et al .: Reactions of Pseudomonas 7A glutaminase-asparaginase with diazo analogues of glutamine and asparagine result in unexpected covalent inhibitions and suggests an unusual catalytic triad Thr-Tyr-Glu , in: Biochem (2000) 39: 1199-1104. doi : 10.1021 / bi991797d
- ↑ a b Eidinoff M et al. Pyrimidine Studies, I. Effect of DON (6-Diazo-5Oxo-L-Norleucine) on incorporation of precursors into nucleic acid pyrimidines (PDF; 867 kB) , 1957.
- ↑ Levenberg B et al. Biosynthesis of the purines, XV. The effect of Aza-L-Serine and 6-Diazo-5-Oxo-L-Norleucine on inosinic acid biosynthesis de novo (PDF; 830 kB) , in: J Biol Chem (1956): 163-176.
- ↑ a b c Ahluwalia GS et al. Metabolism and action of amino acid analog anti-cancer agents , in: Pharmacol Ther (1990) 46: 243-271; PMID 2108451 .
- ↑ Barclay RK et al. Effects of 6-Diazo-5-Oxo-L-Norleucine and other tumor inhibitors on biosynthesis of nicotinamide adenine dinucleotide in mice , in: Cancer Research (1966) 26: 282-286.
- ↑ Rosenbluth RJ et al. DON, CONV and DONV-II. Inhibition of L-Asparagine Synthetase in Vivo , in: Biochemical Pharmacology , Vol. 25, 1851-1858. doi : 10.1016 / 0006-2952 (76) 90189-1 .
- ↑ Wu F et al .: A mechanism behind the antitumor effect of 6-diazo-5-oxo-L-norleucine (DON): disruption of mitochondria. , Eur J Cancer . 1999 Jul; 35 (7): 1155-1161. doi : 10.1016 / S0959-8049 (99) 00099-4 .
- ↑ Hiramoto K et al .: DNA strand cleavage by tumor-inhibiting antibiotic 6-diazo-5-oxo-L-norleucine , Mutat Res . 1996 Jun 10; 360 (2): 95-100. doi : 10.1016 / 0165-1161 (95) 00073-9 .