Ambazon
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Ambazon | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 11 N 7 S | |||||||||||||||
| Brief description |
copper brown crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 237.28 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
193 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ambazon is a medicine that was used as an antiseptic in the mouth and throat . It was patented by Bayer in 1957 and later sold under the trade name Iversal ® , but is no longer on the market today.
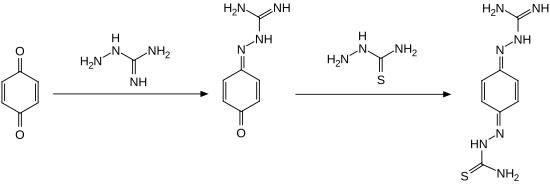
Chemically speaking, ambazone is a functional derivative of 1,4-benzoquinone ( p -benzoquinone). As a derivative of guanidine, the compound has strongly basic properties.
synthesis
The synthesis of ambazone is carried out starting from 1,4-benzoquinone in two stages by reaction with aminoguanidine and thiosemicarbazide :
Individual evidence
- ^ A b Hans Beyer, Wolfgang Walter: Textbook of Organic Chemistry . 20th ed., Hirzel, Stuttgart, 1984. p. 483, ISBN 3-7776-0406-2 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Entry on Ambazon in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Patent DE965723 : Process for the production of condensation products. Registered on January 31, 1953 , published on June 19, 1957 , applicant: Bayer AG , inventor: Siegfried Petersen, Gerhard Domagk .
- ^ A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications , 4th edition (2001) Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 .
Web links
- Entry to Ambazon. In: Römpp Online . Georg Thieme Verlag, accessed on January 2, 2015.