Ammonium perchlorate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 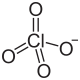
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium perchlorate | |||||||||||||||
| other names |
Superchloric acid ammonium |
|||||||||||||||
| Molecular formula | NH 4 ClO 4 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 117.49 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.95 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
210–240 ° C (decomposition) |
|||||||||||||||
| solubility |
good in water (205.9 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium perchlorate (NH 4 ClO 4 ) is the ammonium salt of perchloric acid (HClO 4 ). It is often made by the action of perchloric acid HClO 4 on ammonium chloride NH 4 Cl.
properties
Ammonium perchlorate ( empirical formula NH 4 ClO 4 ) forms colorless, odorless, easily water-soluble crystals which can explode when exposed to friction, heat and in the presence of strong acids . There is a risk of fire in contact with flammable substances or reducing agents such as sulfur , phosphorus , metal powder and organic substances. In a stabilized state (e.g. with 10% water) the substance is not explosive, but fire-promoting. On contact, severe irritation of the mucous membranes occurs.
The handling of ammonium perchlorate is regulated in the Explosives Act and is prohibited in Germany without a corresponding permit.
Ammonium perchlorate crystallizes orthorhombically , space group Pnma (space group no. 62) with the lattice parameters a = 9.202 Å , b = 5.816 Å and c = 7.449 Å.
Ammonium perchlorate is well soluble in water and polar organic solvents rather poorly.
| Solubility in various solvents at 25 ° C | ||||||||||||
| solvent | water | Methanol | Ethanol | n-propanol | acetone | Ethyl acetate | ||||||
| solubility | in g / 100 g solvent | 24,922 | 6,862 | 1.907 | 0.387 | 2.260 | 0.032 | |||||
use
Ammonium perchlorate mixed with a binding agent is suitable as rocket fuel for solid rockets , fireworks rockets , model rockets or as explosives . The reason for this is that ammonium perchlorate decomposes according to the following equation when ignited or heated above 200 ° C:
- Ammonium perchlorate decomposes into chlorine, oxygen, nitrogen and water when heated above 200 ° C.
Only gaseous reaction products are formed, which expand explosively due to the heat of reaction generated. The ammonium cation acts as a reducing agent , the perchlorate anion as an oxidizing agent . The released oxygen and chlorine can have a further oxidizing effect. Therefore, up to 30% ( mass fraction ) of aluminum is added, which then serves as the actual fuel and, with its high reaction temperature, keeps the reaction between the components of the ammonium perchlorate going. The specific impulse is still lower than with most liquid fuels . Nevertheless, ammonium perchlorate in a mixture with other flammable substances (especially aluminum powder) is used as APCP for rocket boosters , as these are simple and cheap to construct, e.g. for Ariane 5 , Space Shuttle , Titan IIIC-IVB , H-II and Delta - missiles . It is also used in flashing stars (strobe stars), which are occasionally built into large fireworks and fired there in fireworks bombs.
Manufacturing
The industrial production takes place electrolytically in the so-called perchlorate cell . First, sodium chloride NaCl is oxidized in an aqueous solution to sodium perchlorate NaClO 4 . The sodium perchlorate is then reacted with an ammonium salt (for example ammonium chloride NH 4 Cl) in an ion exchange reaction. This creates the end product:
- Sodium perchlorate and ammonium chloride react to form ammonium perchlorate and sodium chloride .
The different solubilities of the salts make them easy to separate. The resulting sodium chloride NaCl can in turn be used as a starting product for the perchlorate cell.
Dangerousness
The uncontrolled burning of ammonium perchlorate is very dangerous:
The crash of South African Airways Flight 295 on November 28, 1987 was caused by a fire in the hold, according to an investigative committee; Among other things, the oxidizing ammonium perchlorate was loaded, but this was not recorded in the official loading papers.
On May 4, 1988, the chemical accident at PEPCON in Henderson, Nevada, resulted in a devastating fire in a factory that manufactured and stored ammonium perchlorate for NASA's rocket fuel . There was an explosion that was felt for more than 25 miles.
In 2015, ammonium perchlorate was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. Ammonium perchlorate intake was driven by concerns about worker exposure , high risk characterization ratio (RCR), other hazard-related concerns and widespread use, and the potential for carcinogenic properties and potential endocrine disruptors . The reassessment took place from 2015 and was carried out by Germany . A final report was then published. Ammonium perchlorate was classified as an endocrine disruptor.
Individual evidence
- ↑ a b c d e f Entry on ammonium perchlorate in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ Entry on ammonium perchlorate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ C. Gottfried, C. Schusterius: The structure of potassium and ammonium perchlorate . In: Zeitschrift für Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie , 84, 1932, pp. 65-73, doi : 10.1524 / zkri.1933.84.1.65 .
- ↑ a b Long, JR: Perchlorate safety: Reconciling inorganic and organic guidelines in Chem. Health Safety 9 (2002) 12-18, doi : 10.1016 / S1074-9098 (02) 00294-0 .
- ↑ Willard, HH; Smith, GF: The Perchlorates of the Alkali and Alkaline Earth Metals and Ammonium. Their Solubility in Water and Other Solvents in J. Am. Chem. Soc. 45 (1923) 286-297, doi : 10.1021 / ja01655a004 .
- ↑ Accident report of the Henderson disaster (PDF, English; 1.62 MB)
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): ammonium perchlorate , accessed on March 26, 2019.
- ↑ Ammonium perchlorate - Endocrine disruptor assessment - ECHA. Retrieved February 21, 2019 (UK English).





