Diphenylmethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diphenylmethane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 13 H 12 | |||||||||||||||
| Brief description |
colorless crystal needles with a fruity, bitter smell |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class |
Anti parasitic agent |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 168.24 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.01 g cm −3 |
|||||||||||||||
| Melting point |
26-28 ° C |
|||||||||||||||
| boiling point |
264 ° C |
|||||||||||||||
| Vapor pressure |
15.1 Pa (50 ° C) |
|||||||||||||||
| solubility |
almost insoluble in water (14 mg l −1 at 25 ° C). |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Diphenylmethane is an organic chemical compound from the group of aromatic hydrocarbons .
Extraction and presentation
Diphenylmethane can be easily produced in laboratory quantities by Grignard addition of phenyl magnesium chloride to benzyl chloride or reduction of benzophenone .
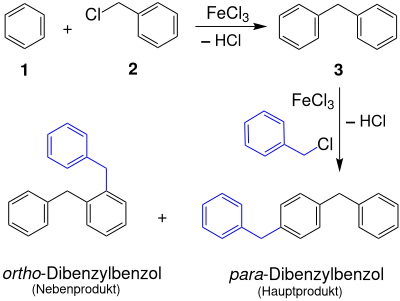
For technical purposes, these methods are uneconomical or difficult to handle. Diphenylmethane ( 3 ) can be obtained by the electrophilic substitution reaction of benzene ( 1 ) with benzyl chloride ( 2 ) in the presence of a Lewis acid such as amalgamated aluminum.
Compared to benzyl chlorides, otherwise conventional aluminum trichloride is poorly suited as a catalyst; Iron compounds such as pyrite or iron (III) chloride are advantageous .
The diphenylmethane formed in this synthesis reaction is then distilled off and purified by recrystallization.
- Dibenzylbenzene
However, a large part is lost through subsequent reactions in the form of - predominantly - para -dibenzylbenzene and remains as a residue that cannot be distilled. This residue can be converted to diphenylmethane with stoichiometric amounts of aluminum trichloride as a "disproportionation catalyst" and excess benzene.
This subsequent reaction, the repeated substitution of benzyl chloride in the para position, leads to “linear polybenzyls”.
Properties and use
Because of its low melting point of 26 ° C, diphenylmethane can be present either as a solid or as a liquid at room temperature, whereby impurities can lower the melting point. As a solid, it is in the form of colorless crystal needles, at temperatures above the melting point as a clear liquid. The compound has a bitter, fruity odor, is flammable, but difficult to ignite.
Diphenylmethane is used as a dielectric liquid in electrical engineering, as a low-volatility solvent for chemical syntheses and dyes , ink carrier when printing with disperse dyes, as an additive in jet fuels, for scenting soap and as a fixative in the odorous industry.
Pharmacological importance
Tricyclic diphenylmethane derivatives were used as antidepressants from the 1960s .
See also
literature
- Beilstein's Handbook of Organ. Chemistry ( benzylbenzene , Syst.Nr. 479), Vol. 5: H 588 , EII 498 .
- Beilstein's Handbook of Organ. Chemistry ( Dibenzylbenzenes , Syst. No. 487), Vol. 5: H 710 , EII 621 .
Individual evidence
- ↑ a b c d e f g h i j Entry for CAS no. 101-81-5 in the GESTIS substance database of the IFA , accessed on December 2, 2015(JavaScript required) .
- ↑ Beilstein (System No. 479), Vol. 5: H 588 , EII 498 .
- ^ WW Hartman and Ross Phillips: Diphenylmethane In: Organic Syntheses . 14, 1934, p. 34, doi : 10.15227 / orgsyn.014.0034 ; Coll. Vol. 2, 1943, p. 232 ( PDF ).
- ↑ G. Drechsler, E. Gerull: Suitability of various FRIEDEL-CRAFTS catalysts for polybenzylbenzene synthesis , in: Journal für Praktische Chemie 26 , 24-31 (1964), doi: 10.1002 / prac.19640260104 .
- ↑ JA Smythe: Use of Iron Pyrites in a Friedel-Crafts' Reaction , in: J. Org. Chem. 121 , 1270-1278 (1922).
- ↑ Use also for isomerization with other arylbenzyls, e.g. B. [1] and [2] .
- ↑ R. Scholl and C. Seer: Splitting off of aromatically bound hydrogen with linking of aromatic nuclei by aluminum chloride, Part 6: Experiments with phenol ethers and with diphenyl methane , in: Reports of the German Chemical Society 55 , 330–341 (1922) , doi: 10.1002 / cber.19220550209 . O-Dibenzylbenzene m.p. 78-79 ° C, p-dibenzylbenzene m.p. 86-87 ° C (p. 336) and JA Smythe.
- ↑ J. Skura, RW Lenz: Lineare Polybenzyle, 6. Polymerization von α-Methylbenzylchlorid , in: Makromolekulare Chemie 182 , 47-58 (1981), doi: 10.1002 / macp.1981.021820107 . - The para substitution is> 70% preferred.
- ↑ List of commercially available dielectrics
- ↑ Patent DE10015840 , filed March 30, 2000.
- ↑ Gustav Ehrhart / Heinrich Ruschig : Arzneimittel , 1968, page 446 f.