Lead (IV) chloride
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
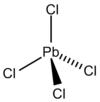
|
||||||||||
| General | ||||||||||
| Surname | Lead (IV) chloride | |||||||||
| Molecular formula | PbCl 4 | |||||||||
| Brief description |
yellow, oily liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 349.012 g · mol -1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
3.18 g cm −3 (at 0 ° C) |
|||||||||
| Melting point |
−15 ° C |
|||||||||
| boiling point |
thermal decomposition: 50 ° C |
|||||||||
| solubility |
hydrolysis in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Thermodynamic properties | ||||||||||
| ΔH f 0 |
329.3 kJ mol −1 |
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Lead (IV) chloride is a chemical compound of the elements lead and chlorine . At room temperature, the substance is an inconsistent, yellow, oily liquid that smokes in the air and decomposes above 50 ° C to form lead (II) chloride and chlorine. Analogous to organic compounds, the PbCl 4 molecules have a tetrahedral structure.
Extraction and presentation
Lead (IV) chloride can be produced by treating lead with chlorine gas or by reacting lead (IV) oxide with concentrated hydrochloric acid:
Another possibility for production is to convert lead (II) chloride with chlorine gas in reverse to the decomposition reaction. However, this is only possible via the hexachloro complex. For this reason, chlorine gas is first introduced into an (ice-cooled) suspension of PbCl 2 in concentrated hydrochloric acid and then lemon-yellow ammonium hexachloroplumbate (NH 4 ) 2 [PbCl 6 ] is precipitated with ammonium chloride . When added to concentrated sulfuric acid , PbCl 4 separates out because it is insoluble in sulfuric acid.
properties
Physical Properties
 Crystal structure of PbCl 4 at 150 K. |
||
| Crystal system | monoclinic | |
| Space group | I 2 / a (No. 15, position 3) | |
|
Lattice parameter (unit cell ) |
a = 1054.2 pm b = 535.9 pm c = 1195.8 pm |
β = 115.83 ° |
| Number (Z) of the formula units |
Z = 4 | |
Chemical properties
Like all other lead (IV) compounds, lead (IV) chloride is a strong oxidizing agent.
In water , lead (IV) chloride hydrolyzes rapidly to lead (IV) oxide and hydrogen chloride:
In connection with a little water a hydrate of unknown composition is formed; with a little cold hydrogen chloride, a solid, crystalline hexachloroblei (IV) acid H 2 [PbCl 6 ] is obtained.
Above 100 ° C there is an explosive decomposition with disproportionation to lead (II) chloride and chlorine .
Individual evidence
- ↑ a b c d e A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1015-1016.
- ↑ Jean D'Ans, Ellen Lax, Roger Blachnik: Paperback for chemists and physicists: Volume 3: elements, inorganic compounds and materials, minerals. 4th edition, Springer, 1998, ISBN 978-3-540-60035-0 , p. 658.
- ↑ a b L. Kolditz: Inorganische Chemie , 2nd edition, Deutscher Verlag der Wissenschaften, Berlin 1983, p. 406.
- ^ Entry on lead compounds in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry lead compounds with the exception of those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) , accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b Pradyot Patnaik: Handbook of inorganic chemicals. McGraw-Hill Professional, 2002, ISBN 978-0-07-049439-8 , pp. 480-481.
- ^ IJ Maley, S. Parsons, CR Pulham: Lead (IV) chloride at 150 K , in: Acta Crystallographica , 2002, No. E 58, pp. I79-i81; doi: 10.1107 / S1600536802015064 .
- ↑ HT Vulte: Laboratory Manual of Inorganic Preparations. Read Books, 2007, ISBN 978-1-4086-0840-1 , p. 40.
- ↑ Bretherick's Handbook of Reactive Chemical Hazards, edited by PG Urben, 6th Edition, Butterworth / Heinemann 1999, ISBN 0-7506-3605-X





![{\ displaystyle \ mathrm {(NH_ {4}) _ {2} [PbCl_ {6}] \ + \ H_ {2} SO_ {4} \ rightarrow (NH_ {4}) _ {2} SO_ {4} + H_ {2} PbCl_ {6}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d1630e3f1692eaa55965cc8145a32700cfe16c0c)


