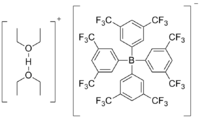
Brookhart's acid
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Brookhart's acid | ||||||||||||
| other names |
Diethyloxoniumethoxyethane tetrakis [3,5-bis (trifluoromethyl) phenyl] borate ( IUPAC ) |
||||||||||||
| Molecular formula | C 40 H 33 BF 24 O 2 | ||||||||||||
| Brief description |
colorless solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 1012.47 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| solubility |
good in diethyl ether |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
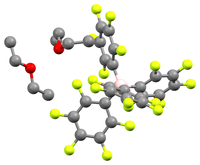
Brookhart's acid refers to the salt of diethyl ether - oxonium ion and tetrakis [3,5-bis (trifluoromethyl) phenyl] borate (BAr F 4 ). It is a colorless solid and is used as a strong acid . The substance was first described by Volpe, Grant and Brookhart in 1992.
presentation
It is prepared by treating NaBAr F 4 in diethyl ether with hydrogen chloride :
- NaBAr F 4 + HCl + 2 Et 2 O → [H (OEt 2 ) 2 ] [BAr F 4 ] + NaCl
While NaBAr F 4 is soluble in diethyl ether, this is not the case with sodium chloride , which is why its precipitation shifts the reaction equilibrium in the direction of oxonic acid . This can be isolated as a solid.
Structure and properties
The acid crystallizes as a colorless, hygroscopic solid. NMR and elemental analysis show that the crystals contain two equivalents of diethyl ether. In solution the substance slowly breaks down into m -C 6 H 3 (CF 3 ) 2 and BAr F 3 .
[H (OEt 2 ) 2 ] [B (C 6 F 5 ) 4 ] is a related compound with a weakly coordinating anion that was published in 2000. An X-ray structure analysis of the compound showed that the acidic proton is coordinated by the two ethereal oxygen atoms, even if the crystal was not good enough to tell whether the proton is symmetrical or asymmetrical.
use
Normally, weakly coordinating anions such as perchlorate , tetrafluoroborate and hexafluorophosphate tend to coordinate to very electrophilic cations , which makes them useless as counterions in some complexes. The highly reactive species [Cp 2 Zr (CH 3 )] + can abstract a fluoride anion from PF 6 , for example . For this reason, new weakly coordinating anions began to be developed in the 1980s. BAr F 4 anions are used as counterions for highly electrophilic, cationic transition metal species because they hardly coordinate and are unreactive to electrophilic attack. A common method for the synthesis of this cationic species is the protonolysis of a dialkyl or olefin complex . The electrophilic palladium catalyst [(2,2'- bipyridine ) Pd ( CH 3 ) ( CH 3 CN )] [BAr F 4 ] is obtained, for example, by protonating the dimethyl compound using Brookhart's acid. The electrophilic, cationic palladium species is in turn used for the polymerization of olefins with carbon monoxide to form polyketones in aprotic solvents .
Potential uses
Polyketones, a group of thermoplastic polymers, are formed by the copolymerization of carbon monoxide with one or more alkenes (typically ethylene or propylene ). Palladium (II) catalysts with a bidentate ligand such as 2,2´-bipyridine or 1,10-phenantroline (phen) and a non-coordinating BAr F counterion, for example [(phen) Pd (CH 3 ) (CO)], are used for this. [BAr F 4 ]. The representation of the catalyst involves the reaction of a dimethyl palladium complex with Brookhart's acid in acetonitrile, whereby methane is split off. In a second step, the acetonitrile is replaced by carbon monoxide.
- [(Et 2 O) 2 H] BAr F 4 + [(phen) Pd (CH 3 ) 2 ] + MeCN → [(phen) Pd (CH 3 ) (MeCN)] BAr F 4 + 2 Et 2 O + CH 4th
- [(phen) Pd (CH 3 ) (MeCN)] BAr F 4 + CO → [(phen) Pd (CH 3 ) (CO)] BAr F 4 + MeCN
The mechanism involves an insertion reaction in which the polymer chain is bound to the catalytic center and grows through sequential insertion of carbon monoxide and the alkene between the palladium atom and the existing chain. Defects (marked in red in the figure below) occur when the insertion does not alternate, i.e. a carbon monoxide insertion is followed by an alkene insertion or an alkene insertion is followed by a carbon monoxide insertion. The above-mentioned catalyst leads to only a few defects, since the difference in the Gibbs energy for the insertion of an alkene directly after an alkene is approximately 12 kJ / mol, which is significantly higher than for the insertion of carbon monoxide.
The use of monodentate phosphine ligands leads to undesirable by-products, but bidentate phosphine ligands such as 1,3-bis (diphenylphosphino) propane have already been used industrially.
Individual evidence
- ↑ a b c d e f M. Brookhart, FC Rix, JM DeSimone, JC Barborak: Palladium (II) catalysts for living alternating copolymerization of olefins and carbon monoxide . In: J. Am. Chem. Soc. . 114, No. 14, 1992, pp. 5894-5895. doi : 10.1021 / ja00040a082 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c d M. Brookhart, B. Grant, AF Volpe: [(3,5- (CF 3 ) 2 C 6 H 3 ) 4 B] - [H (OEt 2 ) 2 ] + : A convenient reagent for generation and stabilization of cationic, highly electrophilic organometallic complexes . In: Organometallics . 11, No. 11, 1992, p. 3920. doi : 10.1021 / om00059a071 .
- ↑ a b Jutzi, P .; Müller, C .; Stammler, A .; Stammler, HG (2000). "Synthesis, Crystal Structure, and Application of the Oxonium Acid [H (OEt 2 ) 2 ] + [B (C 6 F 5 ) 4 ] - ". Organometallics vol. 19, p. 1442. doi : 10.1021 / om990612w
- ↑ I. Krossing, I. Raabe: Noncoordinating anion-Fact or Fiction? A Survey of Likely Candidates . In: Angewandte Chemie International Edition . 43, No. 16, 2004, pp. 2066-2290. doi : 10.1002 / anie.200300620 . PMID 15083452 .
- ↑ RF Jordan, WE Dasher, SF Echols: Reactive cationic dicyclopentadienyl zirconium (IV) complexes . In: Journal of the American Chemical Society . 108, No. 7, 1986, p. 1718. doi : 10.1021 / ja00267a068 .
- ↑ E. Drent, WP Mul, AA Smaardijk: Polyketones . In: Encyclopedia Of Polymer Science and Technology 2001, doi : 10.1002 / 0471440264.pst273 .
- ↑ C. Bianchini, A. Meli: Alternating copolymerization of carbon monoxide and olefins by single-site metal catalysis . In: Coord. Chem. Rev. . 225, No. 1-2, 2002, pp. 35-66. doi : 10.1016 / S0010-8545 (01) 00405-2 .
- ↑ C. Scott Shultz, John Ledford, Joseph M. DeSimone, Maurice Brookhart: Kinetic Studies of Migratory Insertion Reactions at the (1,3-Bis (diphenylphosphino) propane) Pd (II) Center and Their Relationship to the Alternating Copolymerization of Ethylene and carbon monoxide . In: Journal of the American Chemical Society . tape 122 , no. July 27 , 2000, p. 6351-6356 , doi : 10.1021 / ja994251n ( acs.org [accessed October 10, 2019]).
- ^ Francis C. Rix, Maurice Brookhart, Peter S. White: Mechanistic Studies of the Palladium (II) -Catalyzed Copolymerization of Ethylene with Carbon Monoxide . In: Journal of the American Chemical Society . tape 118 , no. January 20 , 1996, p. 4746–4764 , doi : 10.1021 / ja953276t ( acs.org [accessed October 10, 2019]).