Cadmium formate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
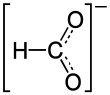 |
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cadmium formate | |||||||||||||||
| other names |
Cadmium diformate |
|||||||||||||||
| Molecular formula | C 2 H 2 CdO 4 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 202.45 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.297 g cm −3 |
|||||||||||||||
| Melting point |
> 350 ° C |
|||||||||||||||
| solubility |
soluble in water (12.3% by mass at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Cadmium formate is a chemical compound of cadmium from the group of carboxylic acid salts with the constitutional formula Cd (HCOO) 2 .
Extraction and presentation
Cadmium formate can be made by dissolving cadmium carbonate in a hot, 15% aqueous solution of formic acid .
properties
Cadmium formate is a white solid that is soluble in water. It decomposes when heated from around 210 ° C, producing carbon dioxide , carbon monoxide , formic acid and cadmium oxide . The compound has a crystal structure with the space group C 2 / c (space group no. 15) . The dihydrate that also exists has a monoclinic crystal structure with the space group P 2 1 / c (No. 14) .
use
Cadmium formate can be used as a catalyst in the vinylation of hydroxy carbon esters and esters of polyhydric alcohols .
Individual evidence
- ↑ a b c d e f g Entry for CAS no. 4464-23-7 in the GESTIS substance database of the IFA , accessed on September 11, 2014(JavaScript required) .
- ↑ a b data sheet cadmium (II) formats, 99.9% trace metals basis from Sigma-Aldrich , accessed on September 11, 2014 ( PDF ).
- ↑ Entry on cadmium diformate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b Barbara Malecka, Agnieszka Lacz: Thermal decomposition of cadmium formats in inert and oxidative atmosphere. In: Thermochimica Acta. 479, 2008, pp. 12-16, doi : 10.1016 / j.tca.2008.09.003 .
- ↑ G. Weber: The structure of anhydrous cadmium formats. In: Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 36, pp. 1947-1949, doi : 10.1107 / S0567740880007595 .
- ↑ Cadmium (II) formats Dihydrate, Acta Cryst. (1974). B30, 1880; BY MICHAEL L. POST AND JAMES TROTTER; Department of Chemistry, University of British Columbia, Vancouver 8, BC, Canada.
- ↑ Peter Müller, Heidi Müller-Dolezal, Renate Stoltz, Hanna Söll, Irmgard Völler: Houben-Weyl Methods of Organic Chemistry Vol. VI / 3, 4th Edition: Ethers… Georg Thieme Verlag, 2014, p. 92 ( limited preview in Google Book search).



