Danazol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
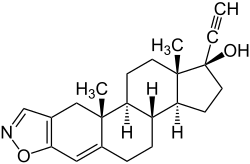
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Danazol | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 22 H 27 NO 2 | |||||||||||||||||||||
| Brief description |
white or pale yellow crystalline powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
Antigonadotropin |
|||||||||||||||||||||
| Mechanism of action |
Inhibition of gonadotropin release |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 337.46 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
224.4-226.8 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Danazol is a derivative of testosterone , as the androgen the Hypophysentätigkeit suppressed, and used to spread the treatment of endometriosis was used. It was patented by Sterling Drug in 1959.
effect
Danazol is a fat-soluble , steroidal derivative of testosterone that reversibly inhibits the release of gonadotropins such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which are produced by the pituitary gland.
As with other androgens, there is also an effect on the telomeres , the ends of the chromosomes . There are indications of an interruption in the mitosis-related reduction in telomere length and even of telomere lengthening under danazol therapy, especially in telomeropathies , hereditary diseases with shortened telomere length due to mutation of individual telomere-associated proteins.
application
Danazol has been used to treat endometriosis since the early 1970s and was approved for this indication in the USA. Danazol causes the uterine lining to develop less and thus reduces the symptoms typical of this clinical picture, such as abdominal pain and irregular bleeding.
Outside of this indication, danazol has been used in the treatment of hereditary angioedema , thrombocytopenic purpura, and fibrotic mastopathy .
In Germany there have no longer been any finished medicinal products containing danazol on the market since 2005, as the manufacturer had waived a subsequent approval due to the unfavorable risk-benefit ratio of the oral dosage form.
Side effects
The side effects are determined by its androgenic properties: By inhibiting the female sex hormones, there is an imbalance in favor of the male. The consequences can be hirsutism , acne, and a deeper voice due to the growth of the larynx, as in a broken voice . While the first two side effects are resolved after stopping the drug, the change in voice can be irreversible.
Trade names
Danatrol (CH), Danokrin (A)
Individual evidence
- ↑ a b c d Entry on Danazol. In: Römpp Online . Georg Thieme Verlag, accessed on May 2, 2014.
- ↑ a b Datasheet Danazol from Sigma-Aldrich , accessed on March 24, 2011 ( PDF ).
- ↑ Danielle M. Townsley, Bogdan Dumitriu, Delong Liu, Angélique Biancotto, Barbara Weinstein, Christina Chen, Nathan Hardy, Andrew D. Mihalek, Shilpa Lingala, Yun Ju Kim, Jianhua Yao, Elizabeth Jones, Bernadette R. Gochuico, Theo Heller, Colin O. Wu, Rodrigo T. Calado, Phillip Scheinberg, Neal S. Young: Danazol Treatment for Telomere Diseases . New England Journal of Medicine 2016; Volume 374, Issue 20 of March 19, 2016, pages 1922-1931; DOI: 10.1056 / NEJMoa1515319 .