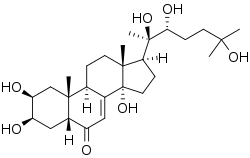
Ecdysterone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ecdysterone | |||||||||||||||
| other names |
20-hydroxyecdysone |
|||||||||||||||
| Molecular formula | C 27 H 44 O 7 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 480.6 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ecdysterone , also called 20-hydroxyecdysone , is a hormone from the group of ecdysteroids , which controls the molting (ecdysis) and metamorphosis of molting animals (ecdysozoa) such as crabs , spiders and insects . In addition, it is formed by many plants as part of their defense against predators. A possible occurrence in mammals results from the ingestion of ecdysterone-containing plants and insects or from an infestation by parasites and can have effects on the mammalian organism. It was already described by H. Hoffmeister in 1966. A contribution to the structure elucidation made H. Hoffmeister, H.-F. Grützmacher and K. Dünnebeil.
biochemistry
Ecdysterone is a steroid hormone derived from cholesterol . It is a C 27 steroid, which differs from the steroid hormones of mammals by a cis connection of the rings A / B and by a pronounced hydroxylation .
biosynthesis
The biosynthesis of ecdysterone in insects was best studied using the example of the fruit fly Drosophila melanogaster . Starting from cholesterol, the first step is the dehydrogenation to 7-dehydrocholesterol with the participation of microsomal cytochrome P450 enzymes in the prothorax gland . In several oxidation steps , the 7-dehydrocholesterol is hydroxylated via ketodiol and ketotriol with the help of various hydroxylases to give 2-deoxyecdysone , ecdysone and finally ecdysterone. The genes which code for the cytochrome P450 enzymes involved are called Halloween genes .
effect
Physiological effect
Ecdysterone acts as a hormone in the representatives of the abundance of molting animals. It regulates numerous physiological processes in molting animals, in particular the molting process, metamorphosis and reproduction. Its hormonal effect is due to an activation of intracellular ecdysone receptors and a consequent specific start of protein biosynthesis , proteins that regulate the moulting process.
Effect on the mammalian organism
Although mammals do not have an ecdysteroid receptor, ecdysterone has potential effects on the mammalian organism. These include influencing protein biosynthesis, fat metabolism and carbohydrate metabolism . For these possible effects, interactions of degradation products of ecdysterone with various nuclear receptors or a direct modulation of GABA receptors are discussed.
In the past, the muscle building effects of ecdysterone were doubted; however, various ecdysteroids - especially ecdysterone - cause anabolic effects in humans ; therefore they have been used for a long time to improve performance in athletes ("mesobolin"). However, a 2018 review summarizes that human studies have failed to show that taking Ecdysterone is anabolic. The harmful side effects of testosterone derivatives do not occur. Since humans can ingest a relevant amount through their food (e.g. from spinach , which contains up to 120 μg / g depending on the variety), ecdysteroids are not yet considered doping substances.
Use as an inductor in research
Ecdysterone and other ecdysteroids are used in biochemical research as inducers in transgenic animals. A new gene is incorporated into an animal in such a way that its expression is under the control of an ecdysone receptor used . Under certain conditions, this offers the possibility of switching the gene used on or off by adding or removing ecdysteroids in the animal's diet ( gene switch ). In addition to the various ecdysteroids, other substances can bind to an ecdysteroid receptor as ligands or coligands, which differ in their suitability as inducers.
In addition to the inducibility, which can be measured according to baseline expression and induction rate, the bioavailability upon oral intake, the reversibility of the binding or induction, and the dose-dependency of the response are important for this function. In particular, however, the respective gene switch system should be specific, i.e. ideally not interfere with endogenous regulatory networks and be able to be activated solely by exogenous connections. For use in animals or humans, only those that do not have a harmful effect on the organism and do not trigger immune reactions appear suitable.
For applications in gene therapy, it makes sense to examine the natural sources of ecdysteroids in humans more closely. In addition to the diet-related phytoecdysteroids, this also seems to include the intestinal flora as well as helminth infections and other diseases. In vitro research suggests that ecdysterone affects some types of blood cells such as lymphocytes and neutrophils and can act as an immunomodulator .
Individual evidence
- ↑ Data sheet 20-Hydroxyecdysone, ≥93% (HPLC), powder from Sigma-Aldrich , accessed on December 3, 2012 ( PDF ).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Nippon Yakurigaku Zasshi. In: Japanese Journal of Pharmacology . Vol. 66, 1970, p. 551.
- ↑ a b L. Dinan, R. Lafont: Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals . In: J. Endocrinol. tape 191 , no. 1 , October 2006, p. 1-8 , doi : 10.1677 / joe.1.06900 , PMID 17065383 .
- ↑ H. Hoffmeister: Ecdysterone, a new metamorphosis hormone of insects. In: Angew Chem Int Ed Engl. 5 (2), Feb 1966, pp. 248-249. PMID 4956560
- ↑ H. Hoffmeister, H.-F. Grützmacher, K. Dünnebeil: Studies on the structure and biochemical action of ecdysterone. In: Z Naturforsch B. 22 (1), Jan 1967, pp. 66-70. PMID 4384828
- ↑ CS Thummel, J. Chory : Steroid signaling in plants and insects - common themes, different pathways . In: Genes Dev. Band 16 , no. 24 December 2002, pp. 3113-3129 , doi : 10.1101 / gad.1042102 , PMID 12502734 .
- ↑ a b MK Parr, F. Botrè, A. Naß, J. Hengevoss, P. Diel: Ecdysteroids: A novel class of anabolic agents? In: Biology of Sport . tape 32 , no. 2 , June 2015, p. 169–173 , doi : 10.5604 / 20831862.1144420 , PMID 26060342 , PMC 4447764 (free full text).
- ↑ S. Tsujiyama, H. Ujihara, K. Ishihara, M. Sasa: Potentiation of GABA-induced inhibition by 20-hydroxyecdysone, a neurosteroid, in cultured rat cortical neurons . In: Jpn. J. Pharmacol. tape 68 , no. 1 , May 1995, pp. 133-136 , PMID 7494377 .
- ↑ RB Kreider, CD Wilborn, L. Taylor et al .: ISSN exercise & sport nutrition review: research & recommendations . In: J Int Soc Sports Nutr . tape 7 , 2010, p. 7 , doi : 10.1186 / 1550-2783-7-7 , PMID 20181066 , PMC 2853497 (free full text).
- ↑ C. Kerksick, C. Wilborn, M. Roberts, A. Smith-Ryan, S. Kleiner: ISSN exercise & sports nutrition review update: research & recommendations . In: Journal of the International Society of Sports Nutrition . tape August 15 , 2018, doi : 10.1186 / s12970-018-0242-y , PMID 30068354 , PMC 6090881 (free full text).
- ↑ a b entry on ecdysteroids. In: Römpp Online . Georg Thieme Verlag, accessed on January 17, 2018.
- ↑ a b R. Lafont and L. Dinan: Practical uses for ecdysteroids in mammals including humans: an update . In: Journal of Insect Science . tape 3 , no. 7 , March 2003, doi : 10.1093 / jis / 3.1.7 , PMID 15844229 , PMC 524647 (free full text).
- ^ E. Saez, MC Nelson, B. Eshelman, E. Banayo, A. Koder, GJ Cho, RM Evans: Identification of ligands and coligands for the ecdysone-regulated gene switch . In: Proceedings of the National Academy of Sciences . tape 97 , no. December 26 , 2000, pp. 14512-14517 , doi : 10.1073 / pnas.260499497 , PMID 11114195 , PMC 18950 (free full text).
- ↑ L. Graham: ecdysone-controlled expression of transgenic . In: Expert Opinion on Biological Therapy . tape 2 , no. 5 , 2002, p. 525-535 , doi : 10.1517 / 14712598.2.5.525 , PMID 12079488 .
- ↑ D. Trenin and V. Volodin: 20-hydroxyecdysone as a human lymphocyte and neutrophil modulator: in vitro evaluation . In: Archives of Insect Biochemistry and Physiology . tape 41 , no. 3 , 1999, p. 156-161 , doi : 10.1002 / (SICI) 1520-6327 (1999) 41: 3 <156 :: AID-ARCH7> 3.0.CO; 2-Q .
