Enzalutamide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
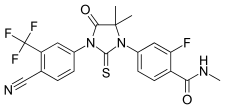
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Enzalutamide | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 21 H 16 F 4 N 4 O 2 S | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
not yet assigned |
|||||||||||||||||||||
| Mechanism of action |
Inhibition of the androgen receptor signaling pathway |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 464.44 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
200-202 ° C |
|||||||||||||||||||||
| solubility | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Enzalutamide is an inhibitor of the androgen receptor signaling pathway. Enzalutamide was developed by Medivation under the code name MDV3100. In 2009, Astellas Pharma and Medivation signed an agreement on the joint further development and global distribution of enzalutamide.
Mechanism of action
Enzalutamide inhibits the androgen receptor signaling pathway in three places. First, it binds to the androgen receptor and thus blocks the binding of natural ligands , e.g. B. testosterone or dihydrotestosterone . Second, enzalutamide inhibits the translocation of the androgen receptor complex into the cell nucleus . And third, it reduces binding to DNA and the recruitment of cofactors for transcription . This threefold mechanism inhibits the expression of the genes that are regulated by the androgen receptor . Enzalutamide thus differs from previously available anti-androgenic substances .
An antagonistic effect on the estrogen receptor in breast cancer cell lines was also observed in laboratory experiments .
Phase III studies
The phase III study AFFIRM started in September 2009 , in which 1199 patients with metastatic castration-resistant prostate cancer were treated with enzalutamide or placebo after prior chemotherapy . The primary endpoint of the study was the overall survival of enzalutamide versus placebo. 800 patients received enzalutamide, 399 patients were in the placebo group. In September 2011, following a planned interim analysis , the study was terminated prematurely on the recommendation of the independent Data Monitoring Committee, as patients with enzalutamide treatment had a 4.8 month longer overall survival than patients in the placebo group (18.4 vs. 6 months; hazard ratio (HR) = 0.63; p <0.001). After unblinding the study, patients in the placebo group were offered enzalutamide as therapy. Enzalutamide was also superior to placebo in the secondary endpoints . The progression-free survival , as determined by radiographic imaging was 5.4 months in the ENZALUTAMIDE group compared with placebo (8.3 months vs. 2.9 months; p <0.001) as well as in PSA -Proof to 5.3 months (8.3 months vs. 3.0 months; p <0.001). 54% of the patients on enzalutamide therapy had a reduction in PSA value of ≥50% compared to the start of the study (p <0.001). 43% of the enzalutamide patients reported an improved quality of life compared to 18% of the placebo patients (p <0.001).
Admission status
In August 2012, the US Food and Drug Administration (FDA) approved enzalutamide for the treatment of metastatic castration-resistant prostate cancer after docetaxel therapy. In June 2013, enzalutamide received approval from the European Medicines Agency (EMA) in Europe for the treatment of patients with metastatic castration-resistant prostate cancer whose disease progresses during or after docetaxel therapy.
Finished medicinal products
- Xtandi (EU, USA)
Individual evidence
- ↑ a b Entry on MDV 3100 at Toronto Research Chemicals , accessed on August 29, 2013 ( PDF ).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Medivation: Astellas and Medivation Enter Into Worldwide Agreement to Co-Develop and Co-Commercialize MDV3100 for the Treatment of Prostate Cancer. (No longer available online.) Archived from the original on August 12, 2013 ; Retrieved July 17, 2013 .
- ↑ C. Tran, S. Ouk, NJ Clegg, Y. Chen, PA Watson, V. Arora, J. Wongvipat, PM Smith-Jones, D. Yoo, A. Kwon, T. Wasielewska, D. Welsbie, CD Chen , CS Higano, TM Beer, DT Hung, HI Scher, ME Jung, CL Sawyers: Development of a second-generation antiandrogen for treatment of advanced prostate cancer. In: Science. Volume 324, number 5928, May 2009, pp. 787-790, doi : 10.1126 / science.1168175 , PMID 19359544 , PMC 2981508 (free full text).
- ↑ Cochrane, DR; Bernales, S; Jacobsen, BM; Cittelly, DM; Howe, EN; d Amato, NC; Spoelstra, NS; Edgerton, SM; Jean, A; Guerrero, J; Gomez, F; Medicherla, S; Alfaro, IE; McCullagh, E; Jedlicka, P; Torkko, KC; Thor, AD; Elias, AD; Protter, AA; Richer, JK (2014). Role of the Androgen Receptor in Breast Cancer and Preclinical Analysis of Enzalutamide . In: Breast Cancer Research 16 (1): R7. doi : 10.1186 / bcr3599 . PMID 24451109 . PMC 3978822 (free full text).
- ↑ HI Scher, K. Fizazi, F. Saad, ME Taplin, CN Sternberg, K. Miller, R. de Wit, P. Mulders, KN Chi, ND Shore, AJ Armstrong, TW Flaig, A. Fléchon, P. Mainwaring , M. Fleming, JD Hainsworth, M. Hirmand, B. Selby, L. Seely, JS de Bono: Increased survival with enzalutamide in prostate cancer after chemotherapy. In: The New England Journal of Medicine . Volume 367, Number 13, September 2012, pp. 1187-1197, doi : 10.1056 / NEJMoa1207506 , PMID 22894553 .
- ↑ FDA : Enzalutamide (XTANDI Capsules). Retrieved July 17, 2013 .
- ↑ EMA : Xtandi (enzalutamide). Retrieved July 17, 2013 .