Ethinamate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
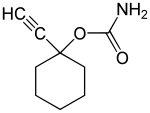
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Ethinamate | ||||||||||||||||||
| other names |
(1-ethynylcyclohexyl) carbamate |
||||||||||||||||||
| Molecular formula | C 9 H 13 NO 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 167.21 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
96 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ethinamate is a drug with short-term sedating and hypnotic effects that has been used to treat insomnia . Regular use leads to tolerance ; the effects usually last no longer than seven days. Ethinamate was patented for Schering in 1957 as a sedative and sleeping aid . It is no longer in trade worldwide.
Presentation and extraction
The synthesis starts from cyclohexanone , which is reacted with ethyne and sodium in liquid ammonia . The carbamate function is then introduced on the resulting 1-ethynylcyclohexanol by reaction with phosgene and ammonia.
Legal status
In the Federal Republic of Germany, ethinamate is a marketable narcotic drug , but not a prescription drug, according to Annex II to Section 1 Paragraph 1 of the Narcotics Act. Possession without permission from the Federal Opium Agency at the Federal Institute for Drugs and Medical Devices is a criminal offense.
Individual evidence
- ↑ Entry on ethinamate in the DrugBank of the University of Alberta .
- ↑ a b Ethinamate data sheet from Sigma-Aldrich , accessed on October 22, 2016 ( PDF ).
- ^ A b c d e f g A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications , 4th edition (2001) Thieme-Verlag Stuttgart, ISBN 978-1-58890 -031-9 .
- ↑ a b Patent US 2,816,910 (Schering 1957).
- ↑ Narcotics Act 1981, Annex II, as of December 25, 2012.
