Ethiprole
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
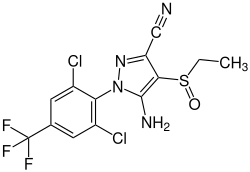
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ethiprole | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 13 H 9 Cl 2 F 3 N 4 OS | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 397.20 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.54 g cm −3 |
||||||||||||||||||
| Melting point |
165.56 ° C (decomposition) |
||||||||||||||||||
| Vapor pressure |
9.1 · 10 −8 Pa (25 ° C) |
||||||||||||||||||
| solubility |
practically insoluble in water (0.0092 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ethiprol is an insecticide from the phenylpyrazole group of active ingredients . It was discovered in 1994 by Bayer CropScience Japan (formerly Rhone-Poulenc Agrochemicals ).
properties
Ethiprole is a non-volatile solid. It is practically insoluble in water, but can be dissolved in organic solvents. Between pH values of 4 and 7, it is stable to hydrolysis for at least 31 days, but slowly degrades at pH 9. The half-life of degradation in the soil is 121 days (calculated value). It is photosensitive in solutions containing water.
Mode of action
The mode of action of Ethiprole is analogous to the effect of the structurally related Fipronil . Ethiprole acts as an antagonist of γ-aminobutyric acid (GABA - abbreviation of English gamma-aminobutyric acid) at the GABA receptor . It causes the receptor to be blocked and no more chloride ions can be exchanged. This leads to overexcitation of the central nervous system , which can lead to paralysis, respiratory failure and death.
Areas of application
Ethiprol is used as an insecticide against biting and sucking pests. As a compound with good systemic activity in plants, it is used as a spray or seed dressing in fruit, rice and vegetable growing.
Analytics
Both liquid and gas chromatographic methods can be used for reliable detection and quantification of ethiprole . A mass spectrometer can be used for identification after the chromatographic separation .
Admission
In the European Union and Switzerland , no pesticides with the active ingredient Ethiprole are approved. The maximum residue limit was set at 0.01 mg / kg for all foods.
Individual evidence
- ↑ a b c d e f g h data sheet Ethiprole PESTANAL, analytical standard at Sigma-Aldrich , accessed on July 23, 2018 ( PDF ).
- ↑ a b Pestice Fact Sheet Ethiprole. (pdf) In: epa.gov. United States Environmental Protection Agency, March 2011, accessed July 19, 2018 .
- ↑ Entry on Ethiprole in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on July 23, 2018.
- ↑ Evaluation Report Ethiprole. (PDF) In: Food Safety Commission Japan. July 21, 2004, accessed March 25, 2019 .
- ↑ FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS .: PESTICIDE RESIDUES IN FOOD 2018 - REPORT 2018JOINT FAO / WHO MEETING ON PESTICIDE RESIDUES. FOOD & AGRICULTURE ORG, [Sl] 2019, ISBN 92-5131156-0 ( limited preview in Google book search [accessed on May 2, 2019]).
- ↑ Simon J. Yu: The Toxicology and Biochemistry of Insecticides, Second Edition . CRC Press, 2014, ISBN 978-1-4822-1061-3 , pp. 147 ( limited preview in Google Book search).
- ↑ Simon J. Yu: The Toxicology and Biochemistry of Insecticides, Second Edition . CRC Press, 2014, ISBN 978-1-4822-1061-3 , pp. 81 ( limited preview in Google Book search).
- ↑ Derick Lucas: Optimizing Sample Preparation for LC / MS / MS of Pesticide Residues in Herbal Teas. (PDF) In: Agilent Technologies, Inc. December 17, 2013, accessed June 28, 2018 .
- ↑ FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS .: PESTICIDE RESIDUES IN FOOD 2018 - REPORT 2018JOINT FAO / WHO MEETING ON PESTICIDE RESIDUES. FOOD & AGRICULTURE ORG, [Sl] 2019, ISBN 92-5131156-0 ( limited preview in Google book search [accessed on May 2, 2019]).
- ^ Directorate-General for Health and Food Safety of the European Commission: Entry on Ethiprole in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on August 1, 2018.

