Expression vector
An expression vector is a nucleic acid that is used to produce recombinant proteins through overexpression . Expression vectors belong to the vectors . In contrast to expression vectors, cloning vectors are not suitable for expressing proteins, but only for cloning .
properties
Expression vectors have an operon so that an mRNA with a protein-coding sequence is generated in a suitable host as an expression system or by cell-free gene expression . A protein is generated from the mRNA in the course of translation . In addition, expression vectors have an origin of replication for the replication of the DNA, a sequence for the selection of the expression vector (e.g. antibiotic resistance , inactivation of a toxic gene or auxotrophy ) and a polylinker into which the protein-coding sequence of the desired protein (the insert ) is inserted. If the protein to be expressed is itself toxic to the expression system, inducible promoters are used which allow the host to grow up to induction. Most often the DNA is used in the form of a plasmid . Often a coding sequence for a protein tag is added to the protein coding sequence, creating a fusion protein that is easier to purify and detect . Expression vectors are often additionally modified by methods of vector design .
Several proteins can also be generated in parallel in a host, provided that plasmids with different selection mechanisms are used, since only one type is necessary with the same selection mechanism and often only one type is retained by the host. Vectors with two promoters are also used. For bacteria, constructs with two cistrons can also be used. In eukaryotes, an IRES can be used to generate two different proteins in parallel between two protein-coding sequences, the yield of the protein with the protein-coding sequence usually being lower after the IRES.
Bacterial Expression Vectors
The cheapest form of protein expression takes place in single cells . However, not all eukaryotic proteins can be produced by bacteria in their native state , as a result of which the biological activity is reduced or undesired inclusion bodies can arise, which have a negative effect on the yield in protein purification. Most Escherichia coli , Bacillus subtilis or, more rarely, Ralstonia eutropha are used as hosts . In addition to plasmids, bacterial artificial chromosomes are also used.
Bacterial expression vectors often have a promoter consisting of the lac operon (then usually the lac UV5 - mutant without catabolite ) or from the bacteriophage T7 (eg the. PET derived vector). Plasmids of the pQE type use a T5 promoter with a lower basal expression , which is better suited for host-toxic proteins. Furthermore, parts of the arabinose- sensitive promoter araBAD or of the rhamnose- sensitive promoter rhaBAD are used to control the induction of gene expression . Promoters composed of different origins are also used, such as the Tac promoter from the trp and the lac promoter, for example in the case of the pGex vector. The origin of replication of plasmids is usually those with a high number of plasmid copies . A high number of plasmid copies leads to high gene expression, but also to higher toxicity of toxic proteins.
If the protein to be expressed has disulfide bridges , a signal sequence for secretion into the periplasm is required because the cytosol has a reducing effect and prevents the formation of disulfide bridges. In most cases, a protease interface is inserted between the signal sequence and the desired protein, whereby the signal sequence is cleaved after or during the purification of the protein in order to obtain a protein that is as native as possible .
Eukaryotic Expression Vectors
The cheapest expression in eukaryotes is carried out in yeasts such as Saccharomyces cerevisiae or Komagataella phaffii (formerly known as Pichia pastoris , as a vector e.g. pIC ), which can glycosylate the proteins. Unless human-like glycosylation patterns are required, one is overexpressed in insect cell culture with baculovirus vectors, or in mammalian cell cultures performed. The vectors used are plasmids, yeast artificial chromosomes (in yeasts), mammalian artificial chromosomes (in mammalian cells) or viral vectors . Although plasmids for use in eukaryotes have a eukaryotic operon, they have a bacterial origin of replication for inexpensive reproduction in bacteria. Due to the lack of eukaryotic origins of replication, plasmids are not replicated in eukaryotes, as a result of which the duration of the expression of the protein is limited because of the degradation of the plasmids ( transient expression ).
In mammalian cells, a CMV immediate / early 1 promoter or an SV40 promoter is often used, more rarely an EF-1 promoter or the chicken β- actin promoter and hybrids of several promoters. Subsequent to the protein-coding sequence, a poly-A sequence (e.g. from the gene for the bovine somatotropin bGH or from the SV40 virus) is used, which slows down the breakdown of the mRNA.
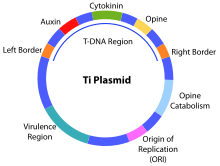
The Ti plasmid is often used in plants, or a viral vector based on the tobacco mosaic virus (TMV), the potato virus X or the cowpea mosaic virus .
use
Expression vectors are used for the production of recombinant proteins, in biochemical research or in the biotechnological-industrial production of proteins. They are also used to generate transgenic organisms on a permanent basis , for gene therapy in humans .
literature
- O. Gileadi: Recombinant Protein Expression in E. coli: A Historical Perspective. In: Methods in molecular biology. Volume 1586, 2017, pp. 3–10, doi : 10.1007 / 978-1-4939-6887-9_1 , PMID 28470595 .
Individual evidence
- ^ T. Terada, T. Murata, M. Shirouzu, S. Yokoyama: Cell-free expression of protein complexes for structural biology. In: Methods in molecular biology. Volume 1091, 2014, pp. 151-159, doi : 10.1007 / 978-1-62703-691-7_10 , PMID 24203330 .
- ^ AK Brödel, DA Wüstenhagen, S. Kubick: Cell-free protein synthesis systems derived from cultured mammalian cells. In: Methods in molecular biology. Volume 1261, 2015, pp. 129-140, doi : 10.1007 / 978-1-4939-2230-7_7 , PMID 25502197 .
- ↑ Dmitriy A. Vinarov, Carrie L. Loushin Newman, Ejan M. Tyler, John L. Markley, Mark N. Shahan: Wheat Germ Cell-Free Expression System for Protein Production. In: Current Protocols in Protein Science (2006). Volume 44, Issue 1, pp. 5.18.1-5.18.18. doi: 10.1002 / 0471140864.ps0518s44 .
- ^ Mary Campbell: Biochemistry. Cengage Learning, 2007, ISBN 978-0-495-39041-1 , p. 378.
- ↑ S. Öztürk, BG Ergün, P. Çalık: Double promoter expression systems for recombinant protein production by industrial microorganisms. In: Applied Microbiology and Biotechnology . Volume 101, Number 20, October 2017, pp. 7459-7475, doi : 10.1007 / s00253-017-8487-y , PMID 28900685 .
- ↑ BE Schoner, RM Belagaje, RG Schoner: Translation of a synthetic two-cistron mRNA in Escherichia coli. In: Proceedings of the National Academy of Sciences . Volume 83, Number 22, November 1986, pp. 8506-8510, PMID 3534891 , PMC 386959 (free full text).
- ↑ IM Terenin, VV Smirnova, DE Andreev, SE Dmitriev, IN Shatsky: A researcher's guide to the galaxy of IRESs. In: Cellular and molecular life sciences: CMLS. Volume 74, number 8, 04 2017, pp. 1431-1455, doi : 10.1007 / s00018-016-2409-5 , PMID 27853833 .
- ↑ GJ Gopal, A. Kumar: Strategies for the production of recombinant protein in Escherichia coli. In: The protein journal. Volume 32, Number 6, August 2013, pp. 419-425, doi : 10.1007 / s10930-013-9502-5 , PMID 23897421 .
- ↑ S. Gruber, D. Schwendenwein, Z. Magomedova, E. Thaler, J. Hagen, H. Schwab, P. Heidinger: Design of inducible expression vectors for improved protein production in Ralstonia eutropha H16 derived host strains. In: Journal of biotechnology. Volume 235, October 2016, pp. 92-99, doi : 10.1016 / j.jbiotec.2016.04.026 , PMID 27085887 .
- ^ AE Silverstone, RR Arditti, B. Magasanik: Catabolite-insensitive revertants of lac promoter mutants. In: Proceedings of the National Academy of Sciences . Volume 66, Number 3, July 1970, pp. 773-779, PMID 4913210 , PMC 283117 (free full text).
- ↑ JW Dubendorff, FW Studier: Controlling basal expression in to inducible T7 expression system by blocking the target T7 promoter with lac repressor. In: Journal of molecular biology. Volume 219, Number 1, May 1991, pp. 45-59, PMID 1902522 .
- ↑ JC Samuelson: Recent developments in difficult protein expression: a guide to E. coli strains, promoters, and relevant host mutations. In: Methods Mol Biol. (2011), Vol. 705, pp. 195-209. PMID 21125387 .
- ↑ J. Konczal, CH Gray: Streamlining workflow and automation to accelerate laboratory scale protein production. In: Protein expression and purification. Volume 133, May 2017, pp. 160-169, doi : 10.1016 / j.pep.2017.03.016 , PMID 28330825 .
- ↑ L. Marschall, P. Sagmeister, C. Herwig: Tunable recombinant protein expression in E. coli: promoter systems and genetic constraints. In: Applied Microbiology and Biotechnology . Volume 101, number 2, January 2017, pp. 501-512, doi : 10.1007 / s00253-016-8045-z , PMID 27999902 , PMC 5566544 (free full text).
- ↑ HA de Boer, LJ Comstock, M. Vasser: The tac promoter: a functional hybrid derived from the trp and lac promoters. In: Proceedings of the National Academy of Sciences . Volume 80, Number 1, January 1983, pp. 21-25, PMID 6337371 , PMC 393301 (free full text).
- ↑ C. French, JM Ward: Production and modification of E. coli transketolase for large-scale biocatalysis. In: Annals of the New York Academy of Sciences. Volume 799, October 1996, pp. 11-18, PMID 8958067 .
- ↑ MP Mayer: A new set of useful cloning and expression vectors derived from pBlueScript. In: Genes. Volume 163, Number 1, September 1995, pp. 41-46, PMID 7557476 .
- ↑ JM Cregg, JL Cereghino, J. Shi, DR Higgins: Recombinant protein expression in Pichia pastoris. In: Molecular biotechnology. Volume 16, Number 1, September 2000, pp. 23-52, doi : 10.1385 / MB: 16: 1: 23 , PMID 11098467 .
- ↑ RD Possee, LA King: baculovirus transfer Vectors. In: Methods in molecular biology. Volume 1350, 2016, pp. 51-71, doi : 10.1007 / 978-1-4939-3043-2_3 , PMID 26820853 .
- ↑ 24312845.
- ↑ KH Khan: Gene expression in Mammalian cells and its applications. In: Advanced pharmaceutical bulletin. Volume 3, number 2, 2013, pp. 257-263, doi : 10.5681 / apb.2013.042 , PMID 24312845 , PMC 3848218 (free full text).
- ↑ Andrew W. Murray, Jack W. Szostak: Construction of artificial chromosomes in yeast. In: Nature. 305, 1983, p. 189, doi : 10.1038 / 305189a0 .
- ^ A. Martella, SM Pollard, J. Dai, Y. Cai: Mammalian Synthetic Biology: Time for Big MACs. In: ACS synthetic biology. Volume 5, number 10, 10 2016, pp. 1040-1049, doi : 10.1021 / acssynbio.6b00074 , PMID 27076218 .
- ^ KL Hefferon: Virus expression vectors. In: Pharmaceutical patent analyst. Volume 3, number 3, May 2014, pp. 249-260, doi : 10.4155 / ppa.14.17 , PMID 24998286 .
- ↑ DW Kim, T. Uetsuki, Y. Kaziro, N. Yamaguchi, S. Sugano: Use of the human elongation factor 1 alpha promoter as a versatile and efficient expression system. In: Gene. Volume 91, Number 2, July 1990, pp. 217-223, PMID 2210382 .
- ↑ L. Xu, H. Mizuguchi, A. Ishii-Watabe, E. Uchida, T. Mayumi, T. Hayakawa: Optimization of transcriptional regulatory elements for constructing plasmid vectors. In: Genes. Volume 272, Numbers 1-2, July 2001, pp. 149-156, PMID 11470520 .
- Jump up ↑ JY Qin, L. Zhang, KL Clift, I. Hulur, AP Xiang, BZ Ren, BT Lahn: Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. In: PLOS ONE (2010), Vol. 5 (5), p. E10611. PMID 20485554 ; PMC 2868906 (free full text).
- ↑ J. Blazeck, HS Alper: Promoter engineering: Recent advances in controlling transcription at the most fundamental level. In: Biotechnol J. (2012), doi : 10.1002 / biot.201200120 . PMID 22890821 .
- ^ R. Walden, J. Schell: Techniques in plant molecular biology-progress and problems. In: European Journal of Biochemistry . Volume 192, Number 3, September 1990, pp. 563-576, PMID 2209611 .
- ↑ MC Cañizares, L. Nicholson, GP Lomonossoff: Use of viral vectors for vaccine production in plants. In: Immunology and cell biology. Volume 83, Number 3, June 2005, pp. 263-270, doi : 10.1111 / j.1440-1711.2005.01339.x , PMID 15877604 .
- ↑ K. Hefferon: Plant Virus Expression Vectors: A Powerhouse for Global Health. In: Biomedicines. Volume 5, number 3, July 2017, p., Doi : 10.3390 / biomedicines5030044 , PMID 28758953 , PMC 5618302 (free full text).