Iron (III) oxide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
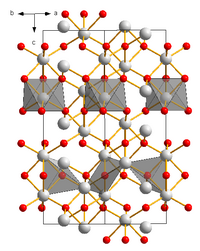
|
|||||||||||||||||||
| __ Fe 3+ __ O 2− | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Iron (III) oxide | ||||||||||||||||||
| other names | |||||||||||||||||||
| Ratio formula | Fe 2 O 3 | ||||||||||||||||||
| Brief description |
red to black crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 159.70 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
5.24 g cm −3 |
||||||||||||||||||
| Melting point |
1539 ° C |
||||||||||||||||||
| solubility |
almost insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
1.5 mg m −3 (aerosol fraction) |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Iron (III) oxide is a stable oxide of iron . Among other things, it is part of the rust and causes its color. It crystallizes in the corundum structure.
Manufacturing
It is possible to obtain red iron oxide by burning iron (III) oxide hydroxide, among other things . During this process, yellow iron oxide is heated to over 200 ° C, with the formation of water vapor.
Meaning and use
Iron (III) oxide is used as a pigment and is known as iron oxide red. The hue varies between red-orange and deep red; it is also the main component of natural red earths .
Iron (III) oxide is used as a magnetizable material as a recording layer for audio tapes . Iron (III) oxide is also a component of superparamagnetic iron oxide nanoparticles , which are used as contrast media in magnetic resonance tomography and especially in molecular imaging .
It is also used in the thermite process in conjunction with aluminum :
- Elemental aluminum reacts with iron (III) oxide to form elemental iron and aluminum (III) oxide .
In the early modern period it was also obtained by distilling iron sulfate (green vitriol) (caput mortuum, head of the dead) and used as red paint.
Occurrence
Iron (III) oxide occurs as a mineral in nature in two modifications :
- Hematite (blood stone) Fe 2 O 3 , trigonal crystal system
- Maghemite ( Maghemite ) Fe 2 O 3 , cubic crystal system
See also
- Iron (II, III) oxide Fe 3 O 4 also: magnetite
- Iron (II) oxide FeO
- Iron (III) oxide hydrate - the hydrate of iron (III) oxide comes e.g. B. as limonite 2Fe 2 O 3 , 3H 2 O or goethite Fe 2 O 3 , H 2 O before.
Web links
Individual evidence
- ↑ Entry on iron oxides. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ a b c d Entry on iron (III) oxide in the GESTIS substance database of the IFA , accessed on August 3, 2007(JavaScript required) .
- ↑ a b Data sheet Iron (III) oxide from Sigma-Aldrich , accessed on March 29, 2011 ( PDF ).
- ↑ about DIN 6164 6 to 8 Kurt Wehlte : Materials and techniques of painting . Otto Maier, Ravensburg 1967, ISBN 3-473-48359-1 (previously: ISBN 3-473-61157-3 ) p. 113.
- ^ Heinrich Schönemann: From the history of chemistry: From the vitriols to sulfuric acid



