Fenoxaprop-P-ethyl
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Fenoxaprop-P-ethyl | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 18 H 16 ClNO 5 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 361.78 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.32 g cm −3 |
||||||||||||||||||
| Melting point |
86.5 ° C |
||||||||||||||||||
| boiling point |
Decomposition from 260 ° C |
||||||||||||||||||
| Vapor pressure |
0.00053 mPa (25 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Fenoxaprop-P-ethyl is a chemical compound from the aryloxyphenoxypropionate group , which was introduced by Hoechst (now Bayer CropScience ).
Extraction and presentation
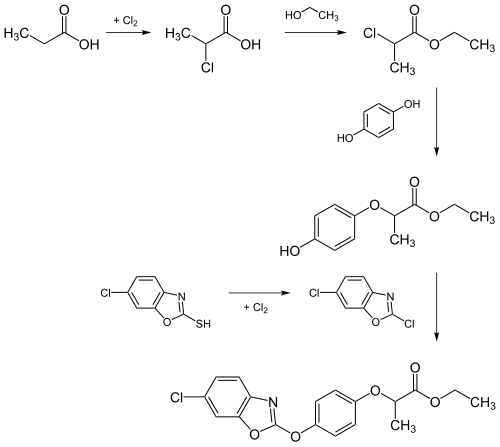
Fenoxaprop-ethyl can be prepared starting from propionic acid . This reacts with chlorine , ethanol , hydroquinone and 2-mercapto-6-chlorobenzoxazole to form the end product.
use
Fenoxaprop-P-ethyl is used as a selective, systemic herbicide against annual monocotyledon weeds such as field foxtail grass , flying oats , millet species and wind stalks , especially in cereal cultivation.
In the cultivation of grain and rice, Fenoxaprop is applied together with the safener Mefenpyr-diethyl (partly also Cloquintocet-mexyl ). In dicotyledon crops such as soybeans, beets, potatoes or rapeseed, it can also be used without a safener.
Fenoxaprop-P-ethyl is an ester that is quickly hydrolyzed to acid, the actual active ingredient, in sensitive plants . It works by inhibiting acetyl-CoA carboxylase (ACCase) in fatty acid biosynthesis.
Admission
Plant protection products with this active ingredient are approved in a number of EU countries, including Austria and Switzerland ( Ralon Super , Puma ).
Individual evidence
- ↑ a b c d e f Entry on Fenoxaprop-P-ethyl in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on March 18, 2014.
- ↑ a b Datasheet Fenoxaprop-P-ethyl, PESTANAL at Sigma-Aldrich , accessed on May 19, 2017 ( PDF ).
- ↑ a b c Entry on Fenoxaprop-P-ethyl in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on (R) -2- [4- (6-chloro-1,3-benzoxazol-2-yloxy) phenoxy] propionic acid ethyl ester in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b entry on fenoxaprop-ethyl. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 768 ( preview ).
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Fenoxaprop-P in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on December 6, 2019.