Hexafluoridosilicic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
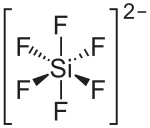
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Hexafluoridosilicic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | H 2 [SiF 6 ] | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 144.09 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.38 g cm −3 (35% solution) |
|||||||||||||||
| Melting point |
−30 ° C (35% solution) |
|||||||||||||||
| boiling point |
110 ° C (decomposition) |
|||||||||||||||
| solubility |
miscible with water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Hexafluoridosilicic acid is a chemical compound that is only known in aqueous solution and has a strong acidic reaction. When attempting to dewater completely, it decomposes into hydrofluoric acid and silicon tetrafluoride . It can be isolated as a dihydrate , which melts at 60 ° C with decomposition. The salts of hexafluoridosilicic acid (e.g. magnesium hexafluoridosilicate Mg [SiF 6 ], potassium hexafluoridosilicate K 2 [SiF 6 ], sodium hexafluoridosilicate Na 2 [SiF 6 ] or zinc hexafluoridosilicate Zn [SiF 6 ]) are called hexafluoridosilicates .
Extraction and presentation
Hexafluoridosilicic acid is formed when hydrofluoric acid reacts with silicon dioxide ( quartz , sand, ...)
or by adding silicon tetrafluoride to water .
It is also formed as a by-product during the digestion (the reaction to produce phosphoric acid) of fluoroapatite with sulfuric acid , whereby hydrofluoric acid is formed, which reacts with the silicates contained in the fluoroapatite in different amounts as an admixture. Since the hexafluoridosilicic acid has to be separated from the phosphorus, it has found various uses as a cheap waste product.
properties
Hexafluoridosilicic acid is a colorless liquid, which in an anhydrous state at room temperature is already about 50% split into silicon tetrafluoride and hydrogen fluoride. It can only be distilled without decomposition as a 13.3% aqueous solution. The hexafluoridosilicate anion SiF 6 2− , unlike the other hexahalidosilicates, is stable to hydrolysis .
use
Hexafluoridosilicic acid is used as a disinfectant or preservative for wooden masts and tanning liquors, for cleaning copper and brass kettles in beer breweries, for acid polishing crystal glass and for the production of metal oxide films. It is also used as a reagent in organic syntheses for splitting Si – O bonds in silyl ethers . In the USA, the compound is often used to fluoridate water (for the purpose of caries prophylaxis). The acid and its salts are used as fluates for the surface treatment of natural and artificial stones ( fluation of concrete ) in order to harden the surface and make it more impermeable to water. They are therefore also known as stone crystallizers . The effect is based on the formation of insoluble fluoride ( calcium fluoride ) from soluble calcium compounds of the material of the surface, as well as silicon dioxide through hydrolysis of the SiF 6 2- anion.
See also
- Hexafluorophosphoric acid HPF 6
- Tetrafluoroboric acid HBF 4
Individual evidence
- ↑ a b c d e f g Entry on hexafluorosilicic acid in the GESTIS material database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on Hexafluorosilicic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Fluorosilicic Acid data sheet (PDF) from Fisher Scientific , accessed February 13, 2014.
- ↑ Entry on fluorosilicic acid. In: Römpp Online . Georg Thieme Verlag, accessed on April 19, 2014.
- ↑ a b G. Brauer (Ed.): Handbook of Preparative Inorganic Chemistry , 2nd ed., Vol. 1, Academic Press 1963, pp. 214-215.
- ↑ e-kunststoffhandel.de: Description of gas scrubbers for the glass industry ( Memento of the original of August 24, 2007 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ Sorglosweb.de: Product description of a hexafluorosilicate mixture ( Memento of the original from February 28, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 93 kB).
- ↑ Otto Hähnle (Ed.): Baustoff-Lexikon . Deutsche Verlags-Anstalt, Stuttgart 1961, p. 333 .


![{\ displaystyle \ mathrm {SiO_ {2} \ + \ 6 \ HF \ \ longrightarrow \ H_ {2} [SiF_ {6}] \ + \ 2 \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f645c090d4a7eb91dd907fcf0af82365bddddc1b)
![{\ displaystyle \ mathrm {3 \ SiF_ {4} \ + \ 2 \ H_ {2} O \ \ longrightarrow \ 2 \ H_ {2} [SiF_ {6}] \ + \ SiO_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5990c267aa3291bc97cc762bc427409a51ad2445)