Flurazole
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
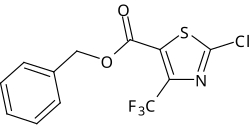
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Flurazole | |||||||||||||||
| other names |
Benzyl 2-chloro-4- (trifluoromethyl) thiazole-5-carboxylate |
|||||||||||||||
| Molecular formula | C 12 H 7 ClF 3 NO 3 S | |||||||||||||||
| Brief description |
|
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 321.70 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
51-53 ° C |
|||||||||||||||
| solubility |
practically insoluble in water: 0.5 mg mg · l −1 at 20 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Flurazole is a chemical compound from the group of thiazoles .
Extraction and presentation
Flurazole, starting from ethyl acetoacetate by reaction with trifluoroacetonitrile , ammonia water , chlorocarbonylsulfenyl chloride , phosphorus oxychloride and sodium hydroxide are obtained.
use
Flurazole is used as a herbicide safener for sorghum . The seed is with 0.06-0.25% flurazole stained what the crop later in front of the herbicides alachlor and metolachlor protects. The compound was introduced by Monsanto in 1994 under the trade name Screen .
Individual evidence
- ↑ a b Entry on flurazole in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on May 2, 2014.
- ↑ a b c Entry on flurazole. In: Römpp Online . Georg Thieme Verlag, accessed on May 4, 2014.
- ↑ a b Entry on flurazole in the GESTIS substance database of the IFA , accessed on May 2, 2014 (JavaScript required)
- ↑ Entry on Benzyl 2-chloro-4- (trifluoromethyl) thiazole-5-carboxylate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 623 ( limited preview in Google Book search).
- ↑ Wolfgang Krämer, Ulrich Schirmer, Peter Jeschke, Matthias Witschel: Modern Crop Protection Compounds . Wiley-VCH, 2011, ISBN 978-3-527-32965-6 , pp. 389–390 ( limited preview in Google Book search).
- ↑ Patent EP0986955A1 : Glutathione conjugates as signaling molecules , Table 9.2 Safeners for sorghum and maize seed treatments .
