Polysaccharides
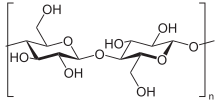
Polysaccharides (also called multiple sugar , glycans / glycans or polyoses hereinafter) are carbohydrates , in which a large number (at least eleven) monosaccharides (simple sugar) via a glycosidic bond , respectively. They are biopolymers made up of at least eleven monosaccharide units or with a statistical distribution of molecular sizes. Examples of polysaccharides are glycogen , starch ( amylose and amylopectin ), pectins , chitin , callose and cellulose . Polysaccharides play an important role in plants and animals as mucilage , reserve substances and nutrients . They can be found in grains and potatoes , for example . Plant cell walls consist of more than 50% cellulose and hemicellulose , the latter is a mixture of polysaccharides that has a supporting function in the cell wall.
Some polysaccharides have the general molecular formula :
- , usually with x equal to 5 or 6 and y = x − 1
Subdivision of the polysaccharides
The polysaccharides are divided into homoglycans (from only one type of simple sugar ) and heteroglycans (from two or more different chain components ) according to the type of individual building blocks ( monomers ) of the molecule . Each monomer building block is connected to the neighboring monomer unit or units via one or more glycosidic bond (s) . A monomer at the end of the chain has at least one neighbor, while chain middle pieces have two neighbors and in the case of branched or subsequently crosslinked chains there can also be three or more neighboring monomers.
Homoglycans
Due to the different linking possibilities, the repeating unit of the polymer, which is enclosed in square brackets in the graphic representation, can also be made up of several identical monosaccharides. Such repeating units from the same monosaccharide are called homodisaccharides for two saccharides and homotrisaccharides for three of the same. Different repeating units from two identical but differently linked monosaccharides have, if they differ only by an α or β link, the same constitution due to the different linkages, but are characterized by different configurations .
This category includes, for example, the two polysaccharides that make up the largest proportion of biomass: starch and cellulose . Both are made up of only one type of monosaccharide . Starch is the main storage form of metabolically active glucose and consists of the two structures amylose and amylopectin , which in turn are made up entirely of α-D-glucose units. Cellulose, on the other hand, has a consistently uniform structure made up of just one monomer, β-D-glucose . Further examples are chitin , the main component of the exoskeletons of arthropods and sinistrin , another plant-based energy store, both of which meet the requirements of homoglycans.
Dextrans are used in medicine as anticoagulants . In cross-linked form, dextrans are used in biochemistry as carrier material in affinity chromatography , pulldown assays and size exclusion chromatography .
Homoglycans, which consist of frequently occurring monomers, are usually divided into subcategories, which are named after the respective monomers. Because of their building blocks, the two polysaccharides cellulose and starch mentioned above belong to the group of glucans . In contrast, Sinistrin, which consists entirely of D-fructose units, is a fructan .
Heteroglycans
As the name suggests ( ancient Greek ἕτερος heteros , 'different', 'different'), the polysaccharides in this category consist of several different monomers , which results in an almost infinite number of possible heteroglycans, which, however, do not all occur in nature.
Heparin and fondaparinux are anticoagulants and representatives of the heteroglycans. Another heteroglycan used primarily in the biochemical laboratory is agarose ; it serves as a medium for agarose gel electrophoresis and for chromatography columns .
Glycosaminoglycans consist of repeating disaccharide units. They are mainly a component of proteoglycans. Heparin and hyaluronic acid are not available in protein-bound form ; the latter is used as a drug in cosmetic surgery and orthopedics .
Biological functions of the polysaccharides
metabolism
For the metabolism , polysaccharides represent an essential energy carrier, whereby representatives of this group of substances serve in most cases as energy stores and are thus products of anabolism . In the animal and human metabolism, glycogen - an analogue of amylopectin - takes on this task. When an organism needs energy, this energy store is released by glucagon , which creates catabolically active D- glucose units. In plants, the homoglycan starch takes on this task.
Glycocalyx
Each cell - both eukaryotic and prokaryotic - is encased in a carbohydrate shell called the glycocalyx . This represents a layer up to 140 nm thick made up of poly- and oligosaccharides . However, these saccharide chains are not unbranched, but are components of glycoproteins and glycolipids, some of which form the cell membrane . The main task of the glycocalyx is - in addition to protecting the cell from drying out - cell-cell communication , which is particularly important for the immune system of an organism .
Polysaccharides are often involved in the structure of the outer shell of certain microorganisms (example: Streptococcus pneumoniae ). Their composition, which can be different within a group of organisms, determines the surface structure and thus the respective serotype when characterizing with different sera .
Proteoglycans
Most of the glycosaminoglycans are involved in the construction of proteoglycans. Hyaluronic acid - a heteroglycan - forms the backbone of the proteoglycans and can be up to 4 µm in length. In addition, this hyaluronic acid chain does not covalently bind the proteins , which in turn covalently associate a large number of glycosaminoglycans - such as heparan , chondroitin and keratan sulfate . Together with collagen and water, proteoglycans build up the cartilage tissue .
Beta glucans
Beta-glucans (β-glucans) are a group of high-molecular β-D-glucose polysaccharides that are found in the cell walls of grain, bacteria and fungi. Depending on the source, they have significantly different physicochemical properties. Cellulose and chitin are also glucans with a β-glycosidic bond. Some β-glucans have anti-tumor and anti-inflammatory effects.
synthesis
Polysaccharides can be produced artificially using the Koenigs-Knorr method .
literature
- Donald Voet, Judith G. Voet and Charlotte W. Pratt: Textbook of Biochemistry . Wiley-VCH, ISBN 978-3-527-32667-9
Individual evidence
- ↑ Entry on glycans . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.G02645 Version: 2.1.5.
- ^ The Free Online Dictionary: polyose
- ↑ Entry on polysaccharides . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.P04752 Version: 2.1.5.
Web links
- Biorama: Biochemistry: Carbohydrates: Polysaccharides ( Memento from December 28, 2011 in the Internet Archive )
![{\ mathrm {[C_ {x} (H_ {2} O) _ {y}] _ {n} \}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a3a7013ff2183d91d1bf03f15a71c0a19d94e4c1)