Guanidinoacetic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Guanidinoacetic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 3 H 7 N 3 O 2 | |||||||||||||||||||||
| Brief description |
White to yellowish, odorless, crystalline solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 117.11 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
300 ° C |
|||||||||||||||||||||
| solubility |
soluble in water (4 g l −1 ) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Guanidinoacetic acid (guanidinoacetate or English acid guanidinoacetic , GAA) is in the body of vertebrates from glycine (a transmission by guanylation guanidine group of arginine formed) and subsequent methylation in creatine converted. It is not an amino acid itself , but it also plays a role in the metabolism of the canonical amino acids serine , threonine and proline . GAA is used as a feed additive in poultry and pig fattening.
presentation
Biochemical synthesis
The formation of guanidinoacetate in the mammalian organism occurs primarily in the kidneys through the transfer of the guanidine group of L-arginine to the amino acid glycine by the enzyme L-Arg: Gly-amidinotransferase (AGAT). Ornithine is produced from L-Arg , which is metabolized to citrulline in the urea cycle by carbamoylation .
In a further step, GAA - in mammals in the liver - is methylated to creatine with S-adenosylmethionine by the enzyme guanidinoacetate-N-methyltransferase (GAMT). The creatine is released into the bloodstream.
Chemical synthesis
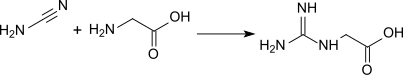
Guanidinoacetic acid was first produced in 1861 by M. Strecker by reacting cyanamide with glycine in an aqueous solution:
Glycine can also be converted to GAA with S-methylisothiourea or with O-alkylisoureas as a guanylating agent.
More recent patent literature describes the synthesis of GAA by catalytic oxidation of ethanolamine to glycine and subsequent reaction with cyanamide in aqueous solution with high yield analogous to the synthesis of creatine starting from N -methylaminoethanol via sarcosine
This route suppresses the formation of the toxicologically questionable dihydrotriazine and other undesirable by-products such as iminodiacetic acid .
properties
Guanidinoacetic acid is obtained as a white (to yellowish) fine powder, which is granulated with starch to improve handling, dosage and absorption to form aggregates with an average diameter of 200-400 µm. The granulate is a long-term stable form of storage for ATMs. The stability of guanidinoacetate in acidic aqueous solution is much higher than that of creatine, which cyclizes to creatinine under acid catalysis .
use
Guanidinoacetic acid is a feed additive approved by EFSA for poultry fattening under the brand name creAMINO®. Even in low doses (600 g / to feed) on a “vegetarian diet”, ie without adding animal protein, it leads to higher feed conversion, weight gain and improved muscle meat should lead.
The advantages of GAA supplementation in other breeding, fattening and domestic animals, as well as the GAA metabolite creatine in high-performance athletes, cannot yet be conclusively assessed. The simultaneous administration of methyl group-supplying substances, such as. B. Betaine appears to be indicated because of the risk of homocysteine formation when GAA is administered alone.
Individual evidence
- ↑ a b Data sheet guanidinoacetic acid from Acros, accessed on February 16, 2013.
- ↑ a b Entry on Glycocyamine in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on February 8, 2013.
- ↑ a b Data sheet Guanidineacetic acid, 99% from Sigma-Aldrich , accessed on February 16, 2013 ( PDF ).
- ↑ Guanidinoacetic acid in piglet feeding - Effects on rearing performance Bavarian State Research Center for Agriculture Institute for Animal Nutrition and Feed Management accessed on May 9, 2020
- ↑ M. Strecker, annual report. Progress Chem. Verw., (1861), 530, doi: 10.1002 / jlac.18611180303 .
- ↑ HI Wheeler, HF Merriam, J. Amer. Chem. Soc., 29 (1903), 478-492.
- ↑ Alzchem: NCN Chemistry News (PDF; 844 kB), edition 1/2011.
- ↑ Patent US8227638 : Process for preparing creatine, creatine monohydrate and guanidinoacetic acid. Published on July 24, 2012 , applicant: Alzchem Trostberg GmbH, inventor: F. Thalhammer, T. Gastner.
- ↑ Patent US2010143703 : Abrasion-resistant free-flowing glycocyamine-containing moldings and methods for their production. Published on June 10, 2010 , inventor S. Winkler et al ..
- ↑ EFSA Scientific Opinion: Safety and efficacy of guanidinoacetic acid as feed additive for chickens for fattening , The EFSA Journal (2009), 988, 1-30.
- ↑ Patent EP1758463 : GUANIDINO ACETIC ACID USED AS AN ANIMAL FOOD ADDITIVE. Published on December 26, 2007 , applicant: Degussa AG, inventor: T. Gastner, H.-P. Krimmer.
- ↑ SM Ostojic et al .: Co-administration of methyl donors along with guanidinoacetic acid reduces the incidence of hyperhomocysteinaemia compared with guanidinoacetic acid administration alone , Br. J. Nutr. (2013), Jan 28: 1-6, doi : 10.1017 / S0007114512005879 .