N -methylethanolamine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | N -methylaminoethanol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 9 NO | |||||||||||||||
| Brief description |
colorless to slightly yellowish liquid with an amine- like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 75.11 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.94 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−5 ° C |
|||||||||||||||
| boiling point |
160 ° C |
|||||||||||||||
| Vapor pressure |
1.06 h Pa (20 ° C) |
|||||||||||||||
| solubility |
miscible with water, ethanol |
|||||||||||||||
| Refractive index |
1.4390 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
N -Methylethanolamine (NMEA, also 2-methylaminoethanol) is a monohydric, primary alcohol as well as a secondary amine and represents a bifunctional synthesis building block for a multitude of secondary products. NMEA is a colorless liquid that smells like amine andreacts basic in water.
Manufacturing
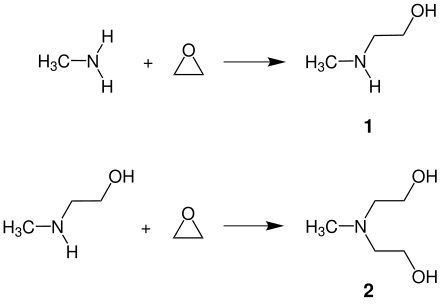
N -methylethanolamine is produced on an industrial scale by reacting ethylene oxide with excess methylamine in aqueous solution. This creates a mixture of the 1: 1 addition product NMEA ( 1 ) and - through further addition of an EO unit - the 1: 2 addition product methyldiethanolamine (MDEA) ( 2 ):
To achieve high yields of the desired target product NMEA, the reactants with a more than twofold excess of methylamine are continuously fed into a flow-through reactor and brought to react while precisely maintaining pressure and temperature. In downstream process steps the excess methylamine and the water are removed and the product mixture is separated into NMEA (bp. 160 ° C) and MDEA (bp. 243 ° C) by fractional distillation. The poly [methyl ethanolamines] formed by the further addition of ethylene oxide to the methylethanolamines formed remain in the distillation bottom.
properties
N -methylethanolamine is a clear, colorless, hygroscopic, amine-like smelling liquid that can be mixed with water and ethanol in any ratio and has a strongly basic reaction in aqueous solution (pH 13.6 at 100 g l −1 (20 ° C )) and is therefore caustic and corrosive. The substance is easily biodegradable and has no bioaccumulation potential due to its water miscibility . NMEA is not mutagenic, but in the presence of nitrite , carcinogenic nitrosamines can be formed from NMEA as a secondary amine . The water hazard class is 1 - slightly hazardous to water. The flash point is 74 ° C, the ignition temperature is 350 ° C and the explosion limits are between 1.5% by volume (lower explosion limit) and 11.7% by volume (upper explosion limit).
use
Like other alkylalkanolamines, N -Methylethanolamine is used in water- and solvent-based paints and coatings as a solubilizer for other components, such as B. Pigments and used as a stabilizer.
In cathodic dip painting , NMEA serves as a cationic dispersant for the epoxy resin during partial neutralization and as a chain extender during the reaction of high molecular weight polyepoxides with polyols .
As a base, NMEA forms neutral salts with fatty acids , which are surface-active soaps with good emulsifying properties and are used in textile and personal care cleaning products. When bleaching cotton-polyester blends, NMEA is used as a brightener.
By methylating N -methylaminoethanol, dimethylaminoethanol and choline [(2-hydroxyethyl) trimethylammonium chloride] are accessible.
Formed in the reaction of long-chain fatty acids with NMEA dehydration N -methyl- N - (2-hydroxyethyl) amides as neutral surfactants are used. Such amides also act as flow improvers and pour point depressants in heavy oils and middle distillates .
The non-proteinogenic amino acid sarcosine is obtained through the catalytic oxidation of NMEA .
N -Methylaminoethanol plays a role as a building block for the synthesis of pesticides and pharmaceuticals, such as B. in the first stage of the reaction sequence to the antihistamine and antidepressant Mianserin (Tolvin®) and to the non-opiodic analgesic Nefopam (Ajan®).
In analogy to aziridine , N- methyl aziridine can be prepared by a Wenker synthesis from NMEA via the sulfuric acid ester or after replacing the hydroxyl group with a chlorine atom,
z. B. by means of thionyl chloride or chlorosulfonic acid to N -methyl-2-chloroethylamine with the help of a strong base (elimination of HCl) in an intramolecular nucleophilic substitution .
Related links
Individual evidence
- ↑ a b c d e f g h i j k Entry on 2-methylaminoethanol in the GESTIS substance database of the IFA , accessed on December 22, 2019(JavaScript required) .
- ^ A b The Dow Chemical Company: Alkyl Alkanolamines , March 2003.
- ↑ Entry on 2-methylaminoethanol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Miura Trading & Finvest Pvt. Ltd .: Methyl Ethanolamines Technology ( Memento of the original from March 6, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 152 kB).
- ↑ Product Safety Assessment, DOW ™ N-Methylethanolamine , The Dow Chemical Company, Version dated March 24, 2010.
- ↑ US patent Re. 30,238 Additives to improve the flow of heavy fuels and crude oils , inventor: EH Specht, JH O'Mara, applicant: Rohm and Haas Co., reissued March 25, 1980.
- ↑ US patent US 8,227,638, Process for preparing creatine, creatine monohydrate and guanidinoacetic acid , inventors: F. Thalhammer, T. Gastner, applicant: Alzchem Trostberg GmbH, issued on July 24, 2012.
- ↑ A. Kleemann et al. J. Engel, Pharmaceutical Ingredients: Synthesis, Patents, Applications , 2nd revised. u. exp. Ed., Georg Thieme Verlag, Stuttgart, New York, 1982, ISBN 3-13-558402-X .
- ↑ JH Drese, The design, synthesis, and characterization of aminosilica adsorbants for CO 2 capture from dilute sources (PDF; 5.3 MB), Ph.D. Thesis, Georgia Institute of Technology, December 2010, p. 175.