CL-20
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
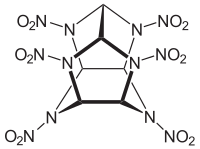
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | CL-20 | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 6 N 12 O 12 | |||||||||||||||
| Brief description |
White dust |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 438.19 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.98 g cm −3 |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
CL-20 , also known as Hexanitroisowurtzitan or HNIW , is one of the most powerful chemical explosives known and belongs to the group of nitramines . The enormous explosive power is due to its high density as well as the high internal tension of the molecule.
Because of the complex synthesis and the associated high production costs, it has hitherto been too expensive for commercial use .
history
CL-20 was discovered in 1987 by researchers from the Naval Air Warfare Center Weapons Division in China Lake, California. Actually, they wanted to make a very strong initial explosive to make a smaller detonator. It is currently the strongest and safest explosive that exists with this detonation speed.
Manufacturing
The synthesis of HNIW takes place in a three-step reaction: First, benzylamine ( 1 ) is allowed to react with glyoxal ( 2 ) under acid catalysis, whereby the hexaazaisowurtzitane cage is built up in a remarkable condensation reaction: the hexabenzyl derivative ( 3 ) is obtained. In the second step, 3 is subjected to a palladium- catalyzed hydrogenation in the presence of acetic anhydride : four of the six benzyl groups are reduced to toluene , and acetyl groups take their place . (The added bromobenzene is used to continuously release small amounts of catalytically active hydrobromic acid ). This gives 4 , which then stepwise first with nitrosyl tetrafluoroborate (NO + BF 4 - then, with) nitronium tetrafluoroborate (NO 2 + BF 4 - to HNIW () 5 ) nitrided is. More recent patents claim that the nitration can also be carried out with much cheaper nitric or nitrating acid .
properties
There are five polymorphs of CL-20 and HNIW : α-HNIW, β-HNIW, γ-HNIW, ε-HNIW and ζ-HNIW. Here, ε-HNIW is the polymorph with the highest crystal density and stability. As an explosive, CL-20 is about 14% stronger than Oktogen , its detonation speed is theoretically 9455 m s −1 . This means that the substance is not superior to the most explosive mixtures of tetranitromethane and toluene (detonation velocities: 9-10 km s −1 ) with a lower density, which in puncture, compression and lead block tests express considerably more energy than octogen. Its explosive power is 1.9 TNT equivalents (explosive power in relation to the explosive power of TNT ). The connection is provided with an impact energy of 4 Nm sensitive to impact , and having a frictional force of 48 N friction sensitive . The thermal stability and vapor pressure of CL-20 are significantly lower than that of Oktogen.
Individual evidence
- ↑ a b c Entry on CL-20. In: Römpp Online . Georg Thieme Verlag, accessed on March 24, 2014.
- ↑ Marguerita Duchoslav, John Priller: Explosives - construction and destruction. ( Memento of November 28, 2012 in the Internet Archive ), Lecture from WS 2006/2007, Didactics of Chemistry, University of Bayreuth (as of September 20, 2010), accessed December 25, 2011.
- ↑ There is not yet a harmonized classification for this substance . A labeling of 2,4,6,8,10,12-HEXANITROHEXAAZA ISOWURTZITANE in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 19, 2019, is reproduced from a self-classification by the distributor .
- ^ TP Russell, PJ Miller, GJ Piermarini, S. Block: Pressure / temperature phase diagram of hexanitrohexaazaisowurtzitane. In: J. Phys. Chem. 97, 1993, pp. 1993-1997, doi: 10.1021 / j100111a043 .
- ↑ Niko Fischer, Dennis Fischer, Thomas M. Klapötke, Davin G. Piercey, Jörg Stierstorfer: Pushing the limits of energetic materials - the synthesis and characterization of dihydroxylammonium 5,5'-bistetrazole-1,1'-diolate . In: Journal of Materials Chemistry . tape 22 , no. 38 , 2012, ISSN 0959-9428 , p. 20418 , doi : 10.1039 / c2jm33646d .
- ↑ J. Köhler, R. Meyer, A. Homburg: Explosivstoffe. 10., completely revised. Edition. Wiley-VCH, Weinheim 2008, ISBN 978-3-527-32009-7 .
Web links
- AT Nielsen et al: Synthesis of polyazapolycyclic caged polynitramines. In: Tetrahedron . 54 (39), 1998, pp. 11793-11812. doi: 10.1016 / S0040-4020 (98) 83040-8 .


