Hexestrol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Structural formula without representation of the stereoisomerism | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Hexestrol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 18 H 22 O 2 | |||||||||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 270.37 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| solubility | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Hexestrol ( INN ) (formerly German Hexöstrol ; also known as Hexanestrol , Hexoestrol and Dihydrodiethylstilbestrol ), is a synthetic , non- steroidal estrogen of the stilbestrol group related to diethylstilbestrol that was used to treat hypoestrogenism but is no longer used medicinally today.
Stereoisomerism
Hexestrol has two similarly substituted stereocenters, consequently there are three stereoisomers, the ( R , R ) form, the ( S , S ) form (also known as sinestrol ) and the meso form. Isohexestrol or DL -hexestrol unspecifically denotes the ( R , R ) form, ( S , S ) form or a mixture of both.
| Isomers of Hexestrol | ||||
| Surname | ( R , R ) -hexestrol | ( S , S ) -hexestrol | meso- hexestrol | |
| other names | Sinestrol | |||
| Isohexestrol DL -hexestrol |
||||
| Structural formula |
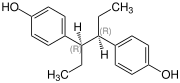
|
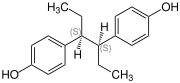
|

|
|
| CAS number | 53625-22-2 | 29555-62-2 | 84-16-2 | |
| 5776-72-7 [( R , R ) or ( S , S )] | ||||
| 5635-50-7 (unspec.) | ||||
| EC number | - | - | 201-518-1 | |
| 227-082-2 (unspec.) | ||||
| ECHA info card | - | - | 100.001.380 | |
| 100.024.621 (unspec.) | ||||
| PubChem | 273497115 | 688058 | 192197 | |
| 234907 [( R , R ) or ( S , S )] | ||||
| 3606 (unspec.) | ||||
| Wikidata | Q61807120 | Q27461267 | ||
| Q27285317 [( R , R ) or ( S , S )] | ||||
| Q5896882 (unspec.) | ||||
Extraction and presentation
Hexestrol is a synthetic hydrogenated derivative of diethylstilbestrol. The hydrogenation of the dimethyl ethers of diethylstilbestrol or pseudodiethylstilbestrol and subsequent demethylation produces the meso isomer, which has a stronger effect than the mixture of isomers. It is also possible to prepare by hydrogenating 4,4'-dihydroxydiphenylhexadiene, by reacting ethylmagnesium bromide with anisaldazine and subsequent demethylation, or starting from p- hydroxypropiophenone.
Hexestrol dimethyl ether can be obtained from anethole hydrobromide by reacting with a Grignard reagent in the presence of a cobalt , nickel or iron halide.
properties
Hexestrol is a white, odorless and tasteless solid that is practically insoluble in water. It is relatively stable at room temperature.
safety instructions
Hexestrol is known to be carcinogenic.
brand names
Synestrol , Synoestrol , Estrifar , Estronal , Hormoestrol , numerous others. The preparations are no longer on the market.
Individual evidence
- ↑ a b c d e f g h Klaus Florey: Profiles of Drug Substances, Excipients and Related Methodology . Academic Press, 1982, ISBN 978-0-08-086106-7 , pp. 360 ( limited preview in Google Book Search).
- ↑ a b c d data sheet Hexestrol, analytical standard at Sigma-Aldrich , accessed on February 8, 2016 ( PDF ).
- ↑ Entry on Hexestrol. In: Römpp Online . Georg Thieme Verlag, accessed on February 8, 2016.
- ↑ IK Morton, Judith M. Hall: Concise Dictionary of Pharmacological Agents: Properties and Definitions . Springer Science & Business Media, December 6, 2012, ISBN 978-94-011-4439-1 , p. 140.
- ^ J. Elks: The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies . Springer, November 14, 2014, ISBN 978-1-4757-2085-3 , pp. 162-163.
- ↑ John A. Thomas: Endocrine Toxicology, Second Edition . CRC Press, March 12, 1997, ISBN 978-1-4398-1048-4 , p. 144.
- ↑ Franz v. Bruchhausen, G. Dannhardt, Siegfried Ebel, August Wilhelm Frahm, Eberhard Hackenthal, Ulrike Holzgrabe: Hager's Handbook of Pharmaceutical Practice Volume 8: Substances EO . Springer-Verlag, 2013, ISBN 978-3-642-57994-3 , pp. 432 ( limited preview in Google Book search).
- ↑ Joachim G. Liehr, Annie M. Ballatore, Beverly B. Dague, Aysegul Ari Ulubelen: Carcinogenicity and metabolic activation of hexestrol. In: Chemico-Biological Interactions . 55, 1985, p. 157, doi: 10.1016 / S0009-2797 (85) 80125-3 .