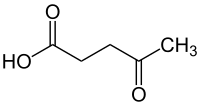
Levulinic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Levulinic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 5 H 8 O 3 | |||||||||||||||||||||
| Brief description |
colorless to yellowish solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 116.11 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.13 g cm −3 |
|||||||||||||||||||||
| Melting point |
33-35 ° C |
|||||||||||||||||||||
| boiling point |
244-246 ° C |
|||||||||||||||||||||
| solubility |
good in water (675 g l −1 at 25 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
The levulinic acid (also 4-oxopentanoic acid or 4-oxovaleric acid ) is a chemical compound belonging to the γ-keto acids is one and the same time represents the simplest representative.
Extraction and presentation
Levulinic acid can be produced in high yield from hexoses such as glucose or fructose by boiling with hydrochloric acid . In addition, levulinic acid can be obtained by hydrolysis of cellulose can be obtained.
use
Levulinic acid is used in textile printing and as a synthesis component in organic syntheses.
Levulinic acid can be used as a bio-based platform chemical . A first practical application are levulinic acid ketals .
In biochemistry , δ-aminolevulinate , a derivative of levulinic acid, is an intermediate product of porphyrin biosynthesis .
Individual evidence
- ↑ Entry on LEVULINIC ACID in the CosIng database of the EU Commission, accessed on July 2, 2020.
- ↑ a b c d e f data sheet levulinic acid (PDF) from Carl Roth , accessed on February 9, 2008.
- ↑ a b c Data sheet levulinic acid (PDF) from Merck , accessed on April 8, 2011.
- ↑ a b Entry on 4-oxopentanoic acid. In: Römpp Online . Georg Thieme Verlag, accessed on August 19, 2015.
- ^ Joseph J. Bozell, Gene R. Petersen: Technology development for the production of biobased products from biorefinery carbohydrates — the US Department of Energy's “Top 10” revisited . In: Green Chemistry . tape 12 , no. January 4 , 2010, doi : 10.1039 / b922014c .
- ↑ Segetis Technology ( Memento of the original from September 12, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.

