Lactoperoxidase
| Lactoperoxidase | ||
|---|---|---|
|
||
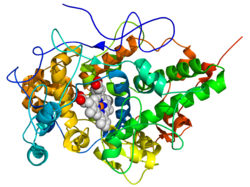
| Ribbon model of the lactoperoxidase of the domestic goat ( Capra hircus ). The protein is shown in rainbow colors ( N-terminus = blue, C-terminus = red) while the heme is shown with spheres (carbon atoms = white, oxygen atoms = red, nitrogen atoms = blue, iron atom = orange). | ||
| Properties of human protein | ||
| Mass / length primary structure | 612 aa | |
| Cofactor | Ca 2+ , heme b | |
| Precursor | (712 aa) | |
| Identifier | ||
| Gene name | LPO | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.11.1.7 , oxidoreductase | |
| Response type | Redox reaction | |
| Substrate | Iodide, bromide, thiocyanate + H 2 O 2 | |
| Products | Hypoiodite, hypobromite, hypothiocyanite + H 2 O | |
| Occurrence | ||
| Parent taxon | Animals | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 4025 | 76113 |
| Ensemble | ENSG00000167419 | ENSMUSG00000009356 |
| UniProt | P22079 | Q5SW46 |
| Refseq (mRNA) | NM_001160102 | NM_080420 |
| Refseq (protein) | NP_001153574 | NP_536345 |
| Gene locus | Chr 17: 58.24 - 58.27 Mb | Chr 11: 87.81 - 87.83 Mb |
| PubMed search | 4025 |
76113
|
The enzyme lactoperoxidase (LPO) is found in most animals and is excreted in humans by the mammary glands , salivary glands and mucous glands of the bronchi . It catalyzes the oxidation of phenols and various anions by hydrogen peroxide . The reaction products are highly reactive molecules that have a toxic effect on microorganisms that have penetrated the body . LPO is therefore part of the innate (unspecific) immune system and enables bacteria in milk and other mucous membrane secretions to be neutralized .
The lactoperoxidase system is a combination of lactoperoxidase and its ionic substrates, hydrogen peroxide and the oxidation products. Well-known substrates are bromide and iodide ions, but also the thiocyanate ion. The products of the enzyme-catalyzed oxidation have a strong antibacterial effect, presumably by inhibiting glucose uptake.
structure
The structure of lactoperoxidase consists mainly of α-helices . There are also two short anti-parallel β-sheets . A heme cofactor is located near the center of the apoprotein .
function
Lactoperoxidase catalyzes the oxidation of various oxygen acceptors by hydrogen peroxide (H 2 O 2 ):
- reduced acceptor + H 2 O 2 → oxidized acceptor + H 2 O
Examples of such oxidation reactions are:
- Thiocyanate (SCN - ) → Hypothiocyanite (OSCN - )
- Bromide (Br - ) → hypobromite (BrO - )
- Iodide (I - ) → hypoiodite (IO - )
The source of hydrogen peroxide is often the reaction of glucose with oxygen in the presence of the enzyme glucose oxidase ( EC 1.1.3.4 ), which also takes place in saliva . The glucose in turn can arise from starch in the presence of the salivary enzyme amyloglucosidase (γ- amylase ( EC 3.2.1.3 )) . Such oxidation products are highly reactive and have a strong antibacterial effect. The lactoperoxidase system is capable of a wide range of aerobic and anaerobic bacteria, including microaerophilic Helicobacter pylori . The effect of the lactoperoxidase system depends on certain experimental conditions. If bacteria are to be cultured on nutrient agar under aerobic conditions after exposure to the lactoperoxidase system, they will not grow. However, they do grow on blood agar under anaerobic conditions. The lactoperoxidase system appears to act synergistically with lactoferrin and lysozyme . If hydrogen peroxide is present in excess of the thiocyanate, the lactoperoxidase system can also have a cytotoxic effect.
Applications
Due to the antibacterial effectiveness of the lactoperoxidase system, it is used to preserve food and cosmetics as well as in ophthalmology. Further applications can be found in the fields of dentistry and wound treatment. The lactoperoxidase system may also be used to fight tumors and viruses.
milk and milkproducts
The lactoperoxidase system inhibits the growth of bacterial flora in milk and dairy products. The addition of hydrogen peroxide and a thiocyanate extends the shelf life of chilled raw milk. The lactoperoxidase system is comparatively thermally stable and is used as an indicator of overpasteurization of milk.
Dentistry
The lactoperoxidase system also appears to be suitable for the treatment of tooth decay , gingivitis and periodontitis and is therefore used as a component in toothpastes and mouthwash solutions. Because it inhibits bacterial growth in the oral cavity, it also inhibits the acid production of these bacteria.
Clinical significance of the lactoperoxidase system
Dental and oral health
In the last few decades a number of clinical studies on the effectiveness of the lactoperoxidase system in various oral hygiene products (toothpastes, mouthwashes) have been published. After it had been shown indirectly via the measurement of experimental gingivitis and caries parameters that mouth rinsing solutions containing amyloglucosidase (γ- amylase ) and glucose oxidase activate the lactoperoxidase system, studies have recently become known that investigate the mechanism of the protective function of enzymes in oral hygiene products. Enzymes such as lysozyme , lactoperoxidase and glucose oxidase are transferred from the toothpaste to the pellicle . As part of the pellicle, these enzymes are highly catalytically active. As a component of toothpaste, the lactoperoxidase system also has a beneficial effect on preventing early childhood caries by reducing the number of colonies of cariogenic microflora that have formed, while at the same time increasing the thiocyanate concentration. Toothpastes with the lactoperoxidase system proved in xerostomia -Patients compared to fluoridated toothpastes terms plaque formation and gingivitis as superior. Further studies of this kind should follow, not least to shed light on the mechanism of the protective effect, which has not yet been fully elucidated. The use of lactoperoxidase is not limited to tooth decay, gingivitis and periodontitis . A combination of lysozyme and lactoperoxidase can also be used to support the treatment of Burning Mouth Syndrome ( glossodynia ). In combination with lactoferrin, lactoperoxidase is effective against bad breath ; in combination with lactoferrin and lysozyme, lactoperoxidase helps relieve symptoms of dry mouth (xerostomia). Likewise, gels with lactoperoxidase can help relieve symptoms in oral cancer patients whose saliva flow is restricted as a result of radiation (xerostomia) and at the same time have a beneficial effect on the bacterial flora .
Cystic fibrosis
Less thiocyanate is found in the saliva of patients with cystic fibrosis than in healthy patients. Because hypothiocyanite with less antibacterial activity can be formed in this way, this could be one reason why these patients suffer more often from respiratory diseases.
cancer
Antibody conjugates with lactoperoxidase kill tumor cells. Macrophages that have been exposed to lactoperoxidase can neutralize tumor cells to a greater extent. The oxidation of estradiol by lactoperoxidase is believed to be a potential source of oxidative stress in breast cancer . Also, estrogen is oxidized in the presence of lactoperoxidase. This creates a reactive phenoxy radical on the phenolic A ring of the estrogen. The action of lactoperoxidase could activate carcinogenic amines in such a way that they react more intensively with DNA and thus contribute to the formation of breast cancer.
Individual evidence
- ↑ a b PDB 2r5l ; Singh, AK, Singh, N., Sharma, S., Kaur, P., Srinivasan, A., Singh, TP: Crystal structure of lactoperoxidase at 2.4 Å resolution . In: J. Mol. Biol. . 376, No. 1, September 2007, pp. 1060-1075. doi : 10.2210 / pdb2r5l / pdb . PMID 18191143 .
- ↑ Thomas EL, Bates KP, Jefferson MM: Hypothiocyanite ion: detection of the antimicrobial agent in human saliva . In: J Dent Res . 59, No. 9, September 1980, pp. 1466-72. doi : 10.1177 / 00220345800590090201 . PMID 6931123 .
- ↑ Tenovuo JO: The peroxidase system in human secretions . In: Tenovuo JO, Pruitt KM (Ed.): The Lactoperoxidase system: chemistry and biological significance . Dekker, New York 1985, ISBN 0-8247-7298-9 , p. 272.
- ↑ Dull TJ, Uyeda C, Strosberg AD, Nedwin G, Seilhamer JJ: Molecular cloning of cDNAs encoding bovine and human lactoperoxidase . In: DNA Cell Biol . 9, No. 7, September 1990, pp. 499-509. doi : 10.1089 / dna.1990.9.499 . PMID 2222811 .
- ↑ Kiser C, Caterina CK, Engler JA, Rahemtulla B, Rahemtulla F: Cloning and sequence analysis of the human salivary peroxidase-encoding cDNA . In: Genes . 173, No. 2, September 1996, pp. 261-4. doi : 10.1016 / 0378-1119 (96) 00078-9 . PMID 8964511 .
- ↑ Kohler H, Jenzer H: Interaction of lactoperoxidase with hydrogen peroxide. Formation of enzyme intermediates and generation of free radicals . In: Free Radic Biol Med . 6, No. 3, 1989, pp. 323-39. doi : 10.1016 / 0891-5849 (89) 90059-2 . PMID 2545551 .
- ↑ Pruitt KM, tab B: Biochemistry of peroxidase systems: antimicrobial effects . In: Tenovuo JO, Pruitt KM (Ed.): The Lactoperoxidase system: chemistry and biological significance . Dekker, New York 1985, ISBN 0-8247-7298-9 , p. 272.
- ↑ Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, Gerson C, Cobas MA, Forteza R, Salathe M, Conner GE: Lactoperoxidase and human airway host defense . In: Am. J. Respir. Cell Mol. Biol. , 29, No. 2, August 2003, pp. 206-12. doi : 10.1165 / rcmb.2002-0152OC . PMID 12626341 .
- ↑ Conner GE, Salathe M, Forteza R: Lactoperoxidase and hydrogen peroxide metabolism in the airway . In: Am. J. Respir. Crit. Care Med . 166, No. 12 Pt 2, December 2002, pp. S57-61. doi : 10.1164 / rccm.2206018 . PMID 12471090 .
- ↑ Conner GE, Wijkstrom-Frei C, Randell SH, Fernandez VE, Salathe M: The Lactoperoxidase System Links Anion Transport To Host Defense in Cystic Fibrosis . In: FEBS Lett . . 581, No. 2, January 2007, pp. 271-8. doi : 10.1016 / j.febslet.2006.12.025 . PMID 17204267 . PMC 1851694 (free full text).
- ↑ a b Tenovuo JO, Pruitt KM (Ed.): The Lactoperoxidase system: chemistry and biological significance . Dekker, New York 1985, ISBN 0-8247-7298-9 , p. 272.
- ↑ Loimaranta V, Tenuovo J, Korhonen H: Combined inhibitory effect of bovine immune whey and peroxidase-generated hypothiocyanite against glucose uptake by Streptococcus mutans . In: Oral Microbiol Immunol . 13, No. 6, 1998, pp. 378-381. PMID 9872115 .
- ↑ a b de Wit JN, van Hooydonk ACM: Structure, functions and applications of lactoperoxidase in natural antimicrobial systems . In: Netherlands Milk & Dairy Journal . 50, 1996, pp. 227-244.
- ↑ Wever R, Kast WM, Kasinoedin JH, Boelens R: The peroxidation of thiocyanate catalysed by myeloperoxidase and lactoperoxidase . In: Biochim. Biophys. Acta . 709, No. 2, December 1982, pp. 212-9. doi : 10.1016 / 0167-4838 (82) 90463-0 . PMID 6295491 .
- ↑ Pruitt KM, Tenovuo J, Andrews RW, McKane T: Lactoperoxidase-catalyzed oxidation of thiocyanate: polarographic study of the oxidation products . In: Biochemistry . 21, No. 3, February 1982, pp. 562-7. doi : 10.1021 / bi00532a023 . PMID 7066307 .
- ↑ Thomas EL, Aune TM: Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action . In: Infection and Immunity . 20, No. 2, May 1978, pp. 456-63. PMID 352945 . PMC 421877 (free full text).
- ↑ Fweja LW, Lewis MJ, Grandison AS: Challenge testing the lactoperoxidase system against a range of bacteria using different activation agents . In: J. Dairy Sci. . 91, No. 7, July 2008, pp. 2566-74. doi : 10.3168 / jds.2007-0322 . PMID 18565914 .
- ↑ Courtois P, Majerus P, Labbé M, Vanden Abbeele A, Yourassowsky E, Pourtois M: Susceptibility of anaerobic microorganisms to hypothiocyanite produced by lactoperoxidase . In: Acta Stomatol Belg . 89, No. 3, September 1992, pp. 155-62. PMID 1481764 .
- ↑ Haukioja A, Ihalin R, Loimaranta V, Lenander M, Tenovuo J: Sensitivity of Helicobacter pylori to an innate defense mechanism, the lactoperoxidase system, in buffer and in human whole saliva . In: J. Med. Microbiol . 53, No. Pt 9, September 2004, pp. 855-60. doi : 10.1099 / jmm.0.45548-0 . PMID 15314191 .
- ↑ a b Carlsson J, Edlund MB, Hänström L: Bactericidal and cytotoxic effects of hypothiocyanite-hydrogen peroxide mixtures . In: Infect Immun . 44, No. 3, June 1984, pp. 581-6. PMID 6724690 . PMC 263633 (free full text).
- ↑ tab B: The biological significance of lactoferrin . In: Int J Tissue React . 5, No. 1, 1983, pp. 87-96. PMID 6345430 .
- Jump up ↑ Roger V, Tenovuo J, Lenander-Lumikari M, Söderling E, Vilja P: Lysozyme and lactoperoxidase inhibit the adherence of Streptococcus mutans NCTC 10449 (serotype c) to saliva-treated hydroxyapatite in vitro . In: Caries Res . . 28, No. 6, 1994, pp. 421-8. doi : 10.1159 / 000262015 . PMID 7850845 .
- ↑ W. James Harper: Biological properties of whey components a review . American Dairy Products Institute, Chicago, IL 2000, p. 54.
- ↑ a b Stanislawski M, Rousseau V, Goavec M, Ito H: Immunotoxins containing glucose oxidase and lactoperoxidase with tumoricidal properties: in vitro killing effectiveness in a mouse plasmacytoma cell model . In: Cancer Res . . 49, No. 20, October 1989, pp. 5497-504. PMID 2790777 .
- ↑ a b Lefkowitz DL, Hsieh TC, Mills K, Castro A: Induction of tumor necrosis factor and cytotoxicity by macrophages exposed to lactoperoxidase and microperoxidase . In: Life Sci . . 47, No. 8, 1990, pp. 703-9. doi : 10.1016 / 0024-3205 (90) 90625-2 . PMID 2402192 .
- ↑ Mikola H, Waris M, Tenovuo J: Inhibition of herpes simplex virus type 1, respiratory syncytial virus and echovirus type 11 by peroxidase-generated hypothiocyanite . In: Antiviral Res . . 26, No. 2, March 1995, pp. 161-71. doi : 10.1016 / 0166-3542 (94) 00073-H . PMID 7605114 .
- ↑ Pourtois M, Binet C, Van Tieghem N, Courtois PR, Vandenabbeele A, Thirty L: Saliva can contribute in quick inhibition of HIV infectivity . In: AIDS . 5, No. 5, May 1991, pp. 598-600. doi : 10.1097 / 00002030-199105000-00022 . PMID 1650564 .
- ↑ B. Reiter, Härnulv BG: The preservation of refrigerated and uncooled milk by its natural lactoperoxidase system . In: Dairy Ind. Int. . 47, No. 5, August, pp. 13-19.
- ↑ Zajac M, Glandys J, Skarzynska M, Härnulv G, Eilertsen K: Milk quality preservation by heat treatment or activation of the lactoperoxidase system in combination with refrigerated storage . In: Milk Science . 38, No. 11, 1983.
- ↑ Zajac M, Glandys J, Skarzynska M, Härnulv G, Björck L: Changes in bacteriological quality of raw milk stabilized by activation of its lactoperoxidase system and stored at different temperatures . In: J. Of Food Prot. . 46, No. 12, 1983, pp. 1065-1068.
- ↑ Korhonen H: A new method for preserving raw milk: The lactoperoxidase antibacterial system . In: World Anim. Rev. . 35, 1980, pp. 23-29.
- ^ Marks NE, Grandison AS, Lewis MJ: Use of hydrogen peroxide detection strips to determine the extent of pasteurization in whole milk . In: International Journal of Dairy Technology . 54, No. 1, 2008, pp. 20-22. doi : 10.1111 / j.0134-727X.2001.00008.x .
- ↑ Patent application WO1988002600 : Enzyme-containing bactericidal composition, and dental and wound treatment preparations comprising this composition. Registered on April 21, 1988 , inventor: Poulson OM.
- ↑ Hoogedoorn H: Activation of the salivary peroxidase system: clinical studies . In: Tenovuo JO, Pruitt KM (Ed.): The Lactoperoxidase system: chemistry and biological significance . Dekker, New York 1985, ISBN 0-8247-7298-9 , pp. 217-228.
- ↑ Hugoson A, Koch G, H Thilander, Hooge Dorn H: lactoperoxidase in the prevention of plaque accumulation, gingivitis and dental caries (III) . In: Odont revy . 25, 1974, pp. 69-80. PMID 4522423 .
- ↑ Midda M, Cooksey MV: Clinical use of an enzyme-containing dentifrice . In: J Clin Periodontol . 13, No. 10, 1986, pp. 959-956. PMID 3098804 .
- ↑ Hannig C, Spitzmüller B, Lux HC, Altenburger M, Al-Ahmad A, Hannig M: Efficacy of enzymatic toothpastes for immobilization of protective enzymes in the in situ pellicle . In: Arch Oral Biol . 55, 2010, pp. 463-469. PMID 20417500 .
- ↑ Hannig C, Hannig M, Attin T: Enzymes in the acquired enamel pellicle . In: Eur J Oral Sci . 113, 2005, pp. 2-13. PMID 15693823 .
- ↑ Jyoti S, Shasikiran ND, Reddy VV: Effect of lactoperoxidase system containing toothpaste on cariogenic bacteria in children with early childhood caries . In: J Clin Pediatr Dent . 33, No. 4, 2009, pp. 299-303. PMID 19725235 .
- ^ Van Steenberghe D, Van den Eynde E, Jacobs R, Quirynen M: Effect of a lactoperoxidase containing toothpaste in radiation-induced xerostomia . In: Int J Dent . 44, No. 2, 1994, pp. 133-138. PMID 15693823 .
- ↑ Hannig C, Hannig M, Attin T: Enzymes in the acquired enamel pellicle . In: Eur J Oral Sci . 113, 2005, pp. 2-13. PMID 15693823 .
- ↑ Kirstilä V, Lenander-Lumikari M, Tenuovo J: Effects of a lactoperoxidase-system-containing toothpaste on dental plaque and whole saliva in vivo . In: Acta Odontol Scan . 52, No. 6, 1994, pp. 346-353. PMID 7887144 .
- ↑ Marino R, Torretta S, Capaccio P, Pignataro L, Spadari F: Different therapeutic strategies for burning mouth syndrome: preliminary data . In: J Oral Pathol Med . 39, No. 8, 2010, pp. 611-616. doi : 10.1111 / j.1600-0714.2010.00922.x . PMID 20701667 .
- ↑ Shin K, Yaegaki K, Murata T, Ii H, Tanaka T, Aoyama I, Yamauchi K, Toida T, Iwatsuki K: Effects of a composition containing lactoferrin and lactoperoxidase on oral malodor and salivary bacteria: a randomized, double-blind, crossover, placebo-controlled clinical trial . In: Clin Oral Investig . 15, No. 4, 2011, pp. 485-493. PMID 20512389 .
- ↑ Gil-Montoya JA, Guardia-Lopéz I, Gonzaléz-Moles MA: Evaluation of the clinical efficacy of a mouthwash and oral gel containing the antimicrobial proteins lactoperoxidase, lysozyme and lactoferrin in elderly patients with dry mouth - a pilot study . In: Gerodontology . 25, No. 1, 2008, pp. 3-9. PMID 18194332 .
- ↑ Nagy K, Urban E, Fazwkas O, Thurzo L, Nagy E: Controlled study of lactoperoxidase gel on oral flora and saliva in irradiated patients with oral cancer . In: J Craniofac Surg . 18, No. 5, 2007, pp. 1157-1164. PMID 17912104 .
- ↑ Shahdad SA, Taylor C, Barclay SC, Steeb IN, Preshaw PM: A double-blind, crossover study of Biotène Oralbalance and BioXtra systems as salivary substitutes in patients with post-radiotherapy xerostomia . In: Eur J Cancer Care . 14, No. 4, 2005, pp. 319-326. PMID 16098116 .
- ↑ Matear DW, Barbaro J: Effectiveness of saliva substitute products in the treatment of dry mouth in the elderly: a pilot study . In: JR Soc Promot Health . 125, No. 1, 2005, pp. 35-41. PMID 15712851 .
- ↑ Xu Y, Szép S, Lu Z: The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases . In: Proc. Natl. Acad. Sci. USA . 106, No. 48, December 2009, pp. 20515-9. doi : 10.1073 / pnas.0911412106 . PMID 19918082 . PMC 2777967 (free full text).
- ↑ Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Nauseef WM, Dupuy C, Bánfi B: A Novel Host Defense System of Airways Is Defective in Cystic Fibrosis . In: Am. J. Respir. Crit. Care Med . 175, No. 2, January 2007, pp. 174-83. doi : 10.1164 / rccm.200607-1029OC . PMID 17082494 . PMC 2720149 (free full text).
- ↑ Minarowski Ł, Sands D, Minarowska A, Karwowska A, Sulewska A, Gacko M, Chyczewska E: Thiocyanate concentration in saliva of cystic fibrosis patients . In: Folia Histochem. Cytobiol. . 46, No. 2, 2008, pp. 245-6. doi : 10.2478 / v10042-008-0037-0 . PMID 18519245 .
- ↑ Sipe HJ, Jordan SJ, Hanna PM, Mason RP: The metabolism of 17 alpha-estradiol by lactoperoxidase: a possible source of oxidative stress in breast cancer . In: Carcinogenesis . 15, No. 11, November 1994, pp. 2637-43. doi : 10.1093 / carcin / 11.15.2637 . PMID 7955118 .
- ↑ a b Ghibaudi EM, Laurenti E, Beltramo P, Ferrari RP: Can estrogenic radicals, generated by lactoperoxidase, be involved in the molecular mechanism of breast carcinogenesis? . In: Redox Rep. . 5, No. 4, 2000, pp. 229-35. doi : 10.1179 / 135100000101535672 . PMID 10994878 .
- ↑ Løvstad RA: A kinetic study on the lactoperoxidase catalyzed oxidation of estrogens . In: Biometals . 19, No. 6, December 2006, pp. 587-92. doi : 10.1007 / s10534-006-0002-3 . PMID 16944280 .
- ↑ Gorlewska-Roberts KM, Teitel CH, Lay JO, Roberts DW, Kadlubar FF: Lactoperoxidase -catalyzed activation of carcinogenic aromatic and heterocyclic amines . In: Chemical Research in Toxicology . 17, No. 12, December 2004, pp. 1659-66. doi : 10.1021 / tx049787n . PMID 15606142 .