Cystic fibrosis
| Classification according to ICD-10 | |
|---|---|
| E84 | Cystic fibrosis |
| E84.0 | Cystic fibrosis with pulmonary manifestations |
| E84.1 | Cystic fibrosis with intestinal manifestations |
| E84.8 | Cystic fibrosis with other manifestations |
| E84.9 | Cystic fibrosis, unspecified |
| ICD-10 online (WHO version 2019) | |
Cystic fibrosis (derived from latin mucus , phlegm ', and viscidus , tough' or ' sticky '), and cystic fibrosis ( ZF , English cystic fibrosis , CF ), is an autosomal - recessive inherited metabolic disease .
The cause of this disease is a mutation- induced malfunction of the chloride channels of certain body cells, which changes the composition of all secretions of exocrine glands . The affected cells are not able to draw water into the surrounding tissue by means of osmosis , which increases the water content of the bronchial secretions and the secretions of the pancreas , the liver ( bile ), the internal genital organs and the accessory sex glands as well as the small intestine and the sweat glands is low. The secretions become viscous as a result, and various types of functional disorders can occur in the affected organs.
Cystic fibrosis is therefore a multisystem disease . More than 2000 different mutations are known which can lead to different forms of cystic fibrosis in those affected. Cystic fibrosis is the most common autosomal recessive hereditary disease and the most common lethal genetic disease in the fair-skinned population. Statistically speaking, there is one sick child for every 2000 live births in this population group . There are considerable regional fluctuations in the frequency of the disease.
The first symptoms appear in early childhood. Cystic fibrosis can be diagnosed prenatally. The fatal disease is currently not curable . Medical progress has made it possible to establish new treatment options over the last few decades , through which the mean life expectancy has now been increased considerably to around 40 years. With new, personalized treatment concepts, a further improvement in life expectancy can be expected in the future. The disease is the subject of intense research.
The two terms cystic fibrosis and cystic fibrosis describe different symptoms of the same disease. In German-speaking countries - in contrast to English-speaking countries - the term cystic fibrosis is preferred.
Epidemiology
In Europe, the probability of having a child with cystic fibrosis is around 1 in 2000. There are currently around 8,000 people with cystic fibrosis in Germany. Worldwide there are around 70,000, of which 30,000 each are in Europe and North America. Around 300 children with cystic fibrosis are born in Germany every year. Cystic fibrosis was the first of the hereditary diseases that used to lead to death in children up to the age of 15.
| mutation | Gene segment | Mutation type | class | D [%] | A [%] | CH [%] |
| ΔF508 | Exon 10 | Amino acid deletion | II | 71.8 | 62.9 | 64.1 |
| R553X | Exon 11 | Stop mutation | I. | 2.0 | 1.7 | 3.5 |
| N1303K | Exon 21 | Amino acid substitution | II | 1.8 | 0.6 | 1.6 |
| R347P | Exon 7 | Amino acid substitution | IV | 1.2 | 1.6 | 0.8 |
| G542X | Exon 11 | Stop mutation | I. | 1.2 | 3.3 | 1.6 |
| G551D | Exon 11 | Amino acid substitution | III | 0.9 | 1.2 | 0.2 |
| 1717 1G → A | Intron 10 | Splice mutation | I. | 0.9 | 0.8 | 3.8 |
| 2789 + 5G → A | Intron 14b | Splice mutation | IV | 0.9 | 2.4 | 0.3 |
| 3905insT | Exon 20 | Frameshift mutation | I. | - | - | 4.8 |
The allele frequency in the German population is around 0.02 to 0.025. This number is a measure of the relative abundance of an allele in a population. It follows that around 4% of the population, i.e. one in twenty- fifth, carries a defective CFTR gene. These approximately three million people in Germany alone are (with exceptions) healthy gene carriers who can pass on the mutated allele. In this case one speaks of heterozygous carriers of traits. The probability that two heterozygous bearers of the trait will father a child has a probability of 0.02² = 0.0004 - based on the total population. With a population of 81.2 million people (as of December 2014, Federal Statistical Office), this would correspond to a mathematical 32,000 inhabitants. In epidemiological studies, a value of 1: 3300 was determined for Germany. Viewed worldwide, the incidences of cystic fibrosis are very different. Ireland has the highest probability of having a child with cystic fibrosis in the world at 1: 1800. Finland has the lowest value in Europe at 1: 25,000. For people of African descent, the risk is about 1 in 17,000. People of Asian descent are most unlikely to be born with the condition at around 1 in 100,000. For example, the value in Japan is 1: 350,000.
Different mutations in the CFTR gene are currently statistically recorded in 2019 (as of June 2017). These mutations are unevenly distributed across the entire population. Fewer than 20 mutations account for more than 0.1 percent and only five mutations account for more than 1 percent of the total number of cystic fibrosis. By far the most common mutation is called ΔF508 . It is found in about 2/3 of all CFTR alleles in cystic fibrosis patients. Within Europe, the prevalence decreases from north-west to south-east.
Among the mutations recorded in 2019, non-synonymous mutations , i.e. point mutations in which a different amino acid is coded for, make up the majority with 39.4%. This is followed by frameshift mutations, which are shifts in the reading frame of CFTR on the DNA , with 15.7% and splice mutations with 11.3%. The proportion of nonsense mutations is 8.4%, that of in frame deletions and in frame insertions is 2.1%. Large insertions and deletions have a frequency of 2.6%. Promoter mutations in the CFTR gene, that is, point mutations in the promoter area of CFTR that lead to reduced gene expression of CFTR , are comparatively rare with a proportion of 0.74%. Only 13.3% of the detected mutations in the coding areas of CFTR do not lead to the disease. The type of mutation is still unknown in 6.5%.
Due to the founder effect , some rather rare mutations can be significantly overrepresented in some populations. The founder effect can arise from religious, ethnic or geographic isolation. For example, the stop mutation W1282X occurs in Ashkenazi Jews , the deletion 394delTT in Nordic peoples , the insertion 3905InsT in Switzerland, the amino acid substitution S549R in Bedouins and the splice mutation 3120 + 1G → A on the African continent comparatively frequently in cystic fibrosis patients. The mutation 3905InsT is a special case. It is only common in Switzerland, the Amish community in North America, and Acadians . In Switzerland, it ranks second among CFTR mutations with a frequency of 4.8% . With the Amish even a value of 16.7% is reached and with Acadians 14.3%. The reason for this is the founding effect of emigrants from German-speaking Switzerland who founded the Amish community in the 18th century and also settled in Louisiana . In Germany, the ΔF508 mutation is found in 72% of patients, the remaining 28%, however, show an extremely heterogeneous and varied spectrum, which makes genetic diagnosis difficult. Over 80 different mutations have so far been detected in German cystic fibrosis patients. Since the mutation form influences the course of the disease and increasingly also the treatment options, the clinical prognosis is often difficult to establish with comparatively rare mutation forms in the CFTR gene. There are too few patients and therefore hardly any case studies with the same CFTR genotype. Of the mutations known in 1995, roughly one in three is so rare that it has only been found in a single family so far ( private mutation ). Spontaneous mutations are extremely rare. Only four cases have been described worldwide.
Genetics and molecular biology
The cause of cystic fibrosis are different mutations in the CFTR - gene that in humans on the long arm of chromosome 7 ( locus sitting q31.2). The CFTR gene codes for the protein Cystic Fibrosis Transmembrane Conductance Regulator (CFTR). This gene product functions as a chloride channel in the cell membrane . The change in the gene also changes the protein and the channel function is either absent or restricted. It is therefore a mutation that leads to a loss of function of the protein concerned ( loss-of-function mutation ). The most common mutation in this gene is called ΔF508. ΔF508 denotes the lack of the amino acid phenylalanine ('F' in the one -letter code ) at position 508 in the CFTR protein and affects about seven in ten people with cystic fibrosis.
So far, over 2000 different mutations of the CFTR gene are known, which occur more or less frequently in different populations. A special feature is that in cystic fibrosis, two different mutations of the CFTR gene, i.e. two different alleles of the same gene, can nevertheless lead to the disease. This particular constellation of an autosomal recessive inheritance is called compound heterozygosity .
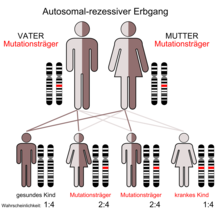
Since cystic fibrosis is inherited in an autosomal recessive manner, the disease only occurs if the carrier of the trait inherits a mutated gene from both parents. If both parents are carriers of one mutated and one unchanged gene, the probability that a child will receive two intact gene copies is 25%. The probability that the child with an intact and a mutated copy is healthy but can pass on the mutation is 50%, and the probability that the child will fall ill, i.e. inherit the disease-causing variant from both parents, is also 25%. If both parents are sick, all children would inherit the disease. However, this is very unlikely as those affected are mostly sterile.
pathology


The CFTR protein, which consists of 1480 amino acids, is synthesized by the endothelial cells in the endoplasmic reticulum in nine to ten minutes . The folding of the highly complex protein takes about 30 to 120 minutes. The folding process is supported by a whole arsenal of chaperones , such as Hsp70 , Hsp40 , Hsp90 and Calnexin . If the protein in this period not correctly folded in front, then it will be chaperones like Hsp70 the ubr1 recruited that the misfolded CFTR ubiquitinyliert and thus the reduction in the 26S proteasome supplies. This protein quality control is a cellular protective mechanism that is of fundamental importance for the maintenance of a functioning proteome and for the survival of the cell. The criterion that decides on degradation in the proteasome and - in the case of CFTR - transport to the cell membrane , is not a reduced function of the protein, but its significantly reduced folding kinetics. Possibly, if the protein had enough time, it would still fold correctly. In fact, about 75% of the CFTR proteins in healthy homozygous people are recognized as "incorrectly" folded by the protein quality control and are broken down.
The CFTR protein is a chloride channel localized on the cell membrane and regulated by cyclic adenosine monophosphate (cAMP), which is mainly expressed by epithelial cells. Depending on the mutation, the expression of the chloride channel can be suppressed, or there are only defective CFTR proteins or CFTR proteins with a restricted function or duration in the cell membrane. The failure of CFTR leads to a disruption of the trans-epithelial transport in all organs in which the epithelial cells express CFTR. These are mainly the respiratory tract , the pancreas, the small intestine, the male reproductive organs and the skin. Cystic fibrosis is therefore a multisystem disease. The failure of CFTR has negative effects mainly on the lung epithelium. In healthy people, on the side facing the breath, this is covered by an approximately 5 µm thick layer, the Airway Surface Liquid (ASL). This layer consists of a thin liquid sol and is slightly less thick than the cilia length (approx. 6 to 7 µm). Viscous mucins that form a gel float on the ASL . This gel has a thickness of one to a few millimeters in healthy people. It represents the first line of defense against inhaled pathogens. It is an elementary component of mucociliary clearance . This line of defense is damaged in patients with cystic fibrosis.
There are two different hypotheses as to why the genetic defect in CFTR leads to the disruption of mucociliary clearance. Both are based on the assumption that the primary defect is the dysfunction of CFTR in ion uptake. The high salt hypothesis assumes that the epithelia no longer absorb sufficient sodium chloride due to the CFTR defect. This leads to an increased concentration of common salt ( high salt ) in the ASL, which in turn severely limits the effect of secreted antimicrobial peptides such as β- defensin . According to the low-volume hypothesis , however, the concentration of sodium chloride in the ASL and in the plasma is the same. However, the failure of CFTR is said to lead to an increased expression of epithelial sodium channels (ENaC), which results in an increased transport of sodium ions into the cell. In order to equalize the osmotic pressure, water molecules then flow from the basolateral side into the epithelia, which leads to a reduction in the volume ( low volume ) of the ASL. The thinner ASL causes a severely restricted mucuciliary clearance, which facilitates the colonization of the mucus with pathogens. Both hypotheses emerged at the end of the 1990s and were discussed controversially for a long time. Different, contradicting treatment options can be derived from both hypotheses. According to the low-volume hypothesis, adding water to the ASL should lead to improvement, while according to the high-salt hypothesis, removing the table salt from the ASL would be the better strategy.
Physiologically, the CFTR protein is more than just a chloride channel on the cell membrane. In addition to the previously mentioned influence on the expression of epithelial sodium channels, CFTR also regulates the Outwardly Rectifying Chloride Channels ( ORCC ) and the Renal Outer Medullary Potassium Channel ( ROMK ). In addition, the chloride-coupled hydrogen carbonate transport and the uptake of sphingolipids are influenced by CFTR.
Mutation classes
The almost 2000 mutations that cause cystic fibrosis can be divided into six classes, which differ in their pathomechanism. Some mutations lead to the almost complete failure of the synthesis of the CFTR protein. In others, for example, the incorporation of the protein into the cell membrane is prevented, or the ion channel of the protein is blocked or has only limited conductivity. With the exception of the group of homozygous patients with the ΔF508 mutation, the other patient groups are comparatively small. Genome-wide association studies (GWAS), which aim to assign a specific genotype to a specific phenotype - in this case the expression of cystic fibrosis - are therefore only possible with a few common CFTR mutation types . Basically, the many different genotypes, for which the homozygous and the compound-heterozygous CFTR variants have to be taken into account, lead to a multitude of different phenotypes with different disease courses, degrees of severity and ultimately also life expectancies.
The classification is not always consistent with the pathomechanism, but sometimes based on the phenotype. The mutation A455E is assigned to class V, for example. Compared to ΔF508, it leads to a milder form of cystic fibrosis. In 2014, however, a working group found that with this mutation a large part of the protein in the proteasome is actually broken down, which - as in the case of ΔF508 - corresponds to a class II mutation. The only difference to ΔF508 is obviously that a smaller proportion of A455E is broken down in the proteasome, which ultimately leads to the milder manifestation of cystic fibrosis in this mutation.
- Class I.
With class I mutations, the genetic defect is so severe that no CFTR protein is produced. The cause for this can be premature stop codons (stop mutations), which lead to an unstable mRNA during transcription . All stop mutations, with the exception of R1162X, belong to class I. The nonsense-mediated mRNA decay (NMD) is a cellular control mechanism that recognizes premature stop codons in the mRNA and prevents their expression as shortened proteins. Examples of class I mutations are Gly542X, Trp1282X, and Arg553X. Another cause can be canonical splice mutations, such as 621 + 1G → T, and chromosomal deletions, such as CFTR del2,3, which also lead to the loss of CFTR production in the endoplasmic reticulum . About 5 to 10% of all cystic fibrosis patients have class I mutations.
- Class II
Protein synthesis does take place in class II mutations, but the primary structure of the CFTR protein is incorrect due to a mutation in the CFTR gene . This affects the tertiary structure of the protein, the protein is incorrectly folded. This protein misfolding is recognized by the protein quality control of the cell, the protein is ubiquitinylated, transported into the proteasome and broken down there. The world's most common CFTR mutation ΔF508 belongs to this class, as do the mutations Asn1303Lys, Ile507del, Arg560Thr and Gly85Glu.
- Class III
In the case of class III mutations, the CFTR protein is produced and also expressed on the cell membrane. However, the chloride channel is not functional because it cannot be opened. Examples of this class are the mutations Gly551Asp, Gly178Arg, Gly551Ser and Ser549Asn. In Germany about 3% of cystic fibrosis patients can be assigned to this mutation class.
- Class IV
Even with class IV mutations, CFTR is expressed on the cell membrane. The chloride channel can be opened, but its permeability for chloride ions is severely restricted. This is the case, for example, with the mutations Arg117His, Arg347Pro, Arg117Cys and Arg334Trp.
- Class V
Class V mutations do not produce enough CFTR protein. The cause for this are mostly mutations in introns , which lead to alternative splicing and thus have a direct negative impact on the amount of CFTR protein produced. Examples of this are the mutations 3849 + 10kbC → T, 2789 + 5G → A and 5T.
- Class VI
Mutation class VI is comparatively rare. Functional CFTR protein is expressed on the cell membrane, but the stability of the protein on the cell membrane is reduced so that it returns to the cytoplasm and is broken down comparatively quickly . An example of this is the 4326delTC mutation. Compared to the wild type , this gene product has a degradation rate that is 5 to 6 times higher.
Clinical picture
Depending on the type of mutation, the symptoms of the disease are more or less pronounced. If the patient has different mutations of the CFTR genes on both chromosomes, only the symptoms of the lesser defect will develop. People with mutations that are less impairing often only have problems with the pancreas ; serious mutations can experience all of the symptoms mentioned.
Respiratory tract


In cystic fibrosis patients, the mucus in the bronchi is significantly more viscous than in healthy people. This leads to chronic coughs , bronchiectasis , frequently recurring lung infections and severe pneumonia . The viscous secretion can only be removed with difficulty by the ciliated epithelium of the trachea and the bronchi. It is therefore a good nutrient medium for pathogens such as Haemophilus influenzae and Staphylococcus aureus , especially in the first few years of the disease, and in the further course Pseudomonas aeruginosa , the Burkholderia- cepacia complex (meaningful: Burkholderia cepacia , B. cenocepacia and B. multivorans) , Stenotrophomonas maltophilia and the "black yeast" ( Exophiala dermatitidis and Exophiala phaeomuriformis ). Those affected often suffer from aspergillosis . At the beginning of the 21st century there was also an increase in the evidence of multi-resistant gram-negative rods and non-tuberculous mycobacteria as well as Inquilinus limosus, Ralstonia species and Pandoraea from the respiratory tract. One consequence of the frequent and protracted lung infections can be increasing lung insufficiency , which becomes noticeable as a chronic lack of oxygen and shortness of breath . Due to the chronic lack of oxygen, the majority of the affected people have watch glass nails and drumstick fingers . The end-stage chronic endobronchial infections lead to cysts , tracheal diverticula , abscesses and pulmonary fibrosis , i.e. to extensive destruction of the airways, which is usually the cause of the early mortality of cystic fibrosis patients. Over 90% of patients die as a result of chronic lower respiratory infections.
Oxidative stress reduces the expression of CFTR in the lungs. In healthy smokers, compared to healthy non-smokers, indications of lower levels of CFTR in the lungs could be found. Oxidative stress, in turn, can be reduced by glutathione , an endogenous antioxidant . It has an important function in protecting the lungs from oxidative stress. The enzyme glutamate cysteine ligase influences the synthesis of glutathione in the cells. A polymorphism in the GCLC gene, which codes for the glutamate cysteine ligase, can cause an increased production of glutathione in the lungs. In a study from 2006, 440 cystic fibrosis patients were examined for the possible influence of GCLC polymorphism on the clinical picture. A significant relationship was found between the functional polymorphism of GCLC and the severity of cystic fibrosis in patients with a CFTR genotype of the mild form.
Digestive tract
In infants, a meconium ileus occurs in 10 to 20% of cases . This is a severe intestinal obstruction ( ileus ) caused by the tough first faeces (meconium) - often the first manifestation of cystic fibrosis. In the elderly, obstruction syndromes due to viscous intestinal secretions are found in 20% . This can lead to a complication in individual cases, the so-called meconium ileus equivalent. It manifests itself in repeated abdominal pain, palpable intestinal contents and an obstruction of the intestine ( obturatio intestini ). These symptoms are accompanied by vomiting.
The function of the pancreas is also disturbed. The lack of secretion results in chronic diarrhea, fatty stools , maldigestion , malnutrition and digestive disorders as well as being underweight . The increasing fibrosis of the pancreas leads to the sinking of the islets of Langerhans , which can lead to pancreatogenic diabetes mellitus . In these cases, one speaks of CF-associated diabetes (CFRD), which is classified as type 3 diabetes mellitus.
About 75% of patients over the age of 19 have impaired glucose tolerance. Even in children, the risk of diabetes mellitus (diabetes) is 10 times higher than in healthy children of the same age. The risk increases with age. Usually an early administration of insulin is recommended, which with its anabolic effect also contributes to the strengthening of the patient.
Affected adults are prone to liver cirrhosis and gallstones due to impaired liver and biliary tract function . Cirrhosis of the liver develops in 5.6% of patients, and gallstones develop in up to 25%.
Skeletal system

The significant improvements in the survival rate achieved in cystic fibrosis patients over the past few decades have led to an increased incidence of late complications, including osteoporosis . About a third of all adult patients with cystic fibrosis also have osteoporosis. These patients have significantly reduced bone density. This increases the risk of fractures and the development of kyphosis ("humps"). Rib fractures are 10 to 100 times more common in adult cystic fibrosis patients than in a healthy comparison group.
The exact causes of the reduced bone density have not yet been adequately researched. It is obviously a very complex interplay of several pathomechanisms. Bad nutritional status, the malabsorption of vitamins D and K , as well as a reduced calcium and phosphate content in the serum play an essential role . Studies have also shown that bone resorption (bone breakdown) is increased during infectious phases and that disturbances in bone formation can be observed. In such phases, the number of osteoclasts responsible for bone loss is significantly increased. More recent research also suggests that the impaired function of the CFTR protein leads to a dysfunction of the osteoblasts responsible for bone formation . Osteoblasts express CFTR. The absence of the chloride channel in osteoblasts obviously leads to disturbances of the equilibrium between osteoprotegerin and prostaglandin E 2 . Long-term use of drugs, especially glucocorticoids to improve the respiratory condition, can also promote bone loss. This is especially the case in cystic fibrosis patients who have had a lung transplant. To suppress organ rejection in the acute phase, you receive glucocorticoids in high, then in low doses.
Reproductive organs
In most cases, sick men are sterile . Although sperm are formed normally, the vas deferens are missing on both sides. This is known as congenital bilateral aplasia of vas deferens (CBAVD for congenital bilateral aplasia of vas deferens ). CBAVD is an independent clinical picture in which 75 to 80% of the men affected carry mutations in the CFTR gene. Those affected may lack all of the other symptoms of cystic fibrosis. Such cases can be viewed as a mild special form of cystic fibrosis. A special mutation spectrum is responsible for the mild expression, especially of the 5T allele. 70 to 80% of men with CAVD have two mutations in the CFTR gene, one of which is a mild allele that causes, for example, only one amino acid change in one transmembrane region.
In women, viscous secretions in the cervical canal reduce fertility. About 50% of the patients are able to conceive. If the overall situation is stable, cystic fibrosis patients can carry a pregnancy to term.
Diagnosis
A possible cystic fibrosis can be detected by means of prenatal diagnostics before birth. It is usually recommended if the family already has a sick child. It can also be indicated for the siblings of a cystic fibrosis patient , the so-called index patient , as well as for the siblings of the heterozygous parents. This also applies to cystic fibrosis patients who wish to have children, for example via in vitro fertilization . For more distant relatives of the index patient there is usually no need for prenatal diagnosis. For them, the risk of having a child with cystic fibrosis is less than the general perinatal mortality . Before the prenatal diagnosis, a detailed clinical-genetic consultation is necessary in order to inform the parents about all risks of the procedure, the illness and the possibilities of an abortion . Before the prenatal diagnosis, a CFTR genotype analysis is also carried out from the blood of the index patient and his or her parents. If the mutation genotype of the index patient is known, prenatal diagnosis using the DNA from fetal material is possible. Two methods are established for collecting samples: the chorionic villus sampling and the amniocentesis (examination of the amniotic fluid). The former is usually carried out in the 10th to 14th and the latter in the 12th to 16th week of pregnancy .
The sweat test is the method of choice for suspected cystic fibrosis in infants and children. The excretion of sweat is stimulated with the help of the medicinal substance pilocarpine . To do this, a weak direct current is applied to the skin, which mediates the diffusion of pilocarpine to the sweat glands of the skin. This procedure is called iontophoresis . A sweat sample is sucked up from the stimulated area with a capillary and the sample is quantitatively analyzed for sodium or chloride content. If the sodium chloride content in the sample is above 80 mmol / l , there is a considerable suspicion of cystic fibrosis. The normal value is in the range of 5 to 55 mmol / l. The sweat test is usually repeated over two days. If it is positive or if the results are inconclusive, a DNA analysis (genetic test) is usually carried out .
A more complex alternative to the sweat test is the determination of immunoreactive trypsin in blood serum . It is usually carried out on the fifth day of life. For this purpose, whole blood is taken from the heel and the trypsin is determined by means of a radioimmunoassay . The normal value is below 80 ng / ml. The results are more meaningful than the sweat test.
The first newborn screening (NGS) programs for cystic fibrosis began in New Zealand and Australia in 1981 . In Switzerland, cystic fibrosis has also been routinely checked for cystic fibrosis as part of newborn screening since 2011. Currently (as of 2013) this is also done in the United States, England, Ireland, Scotland, France, Austria, Poland, the Netherlands and in regions of Italy and Spain.
In Mecklenburg-Western Pomerania , a test for cystic fibrosis has been offered in all newborns as part of the newborn screening for metabolic diseases since 2012 . In 2013, only around 15% of newborns in Germany were examined for cystic fibrosis. Therefore, the disease is only recognized in about 58% of children with cystic fibrosis in the first year of life. The average age at diagnosis is 4.8 years. 7.6% of the patients are 18 years or older at the time of the first diagnosis (as of 2012). Early diagnosis enables timely treatment, which can improve the course and quality of life of the patient. Compared to the clinical diagnosis, one promises a long-term cost advantage. There has therefore been a demand for nationwide newborn screening for years, especially in the medical profession. A gene-based technique is used in the United States, United Kingdom, France and Australia. The German Genetic Diagnostics Act only allows this under strict conditions. This is one of the reasons why the trypsin test is favored in Germany.
An essential criterion for the usefulness of a newborn screening is the principles set by the World Health Organization (WHO) that “it must be a serious disease, the etiology and pathogenesis of which is understood, which becomes manifest after a latent or early symptomatic stage for which it is the medical and organizational possibilities for successful treatment and for which suitable test and examination methods are available ”.
A general heterozygote screening with a eugenic goal is strictly rejected by most geneticists, pediatricians and especially adult cystic fibrosis patients. The detection of heterozygous carriers of traits in the trypsin test is also viewed as problematic. The European Community Concerted Action for Cystic Fibrosis recommends limiting the test for CFTR heterozygosity to subjects with a positive family history.
In 2005, only 6% of the initial diagnosis of 'cystic fibrosis' was found through screening. Most frequently, the diagnosis was based on pulmonary (27.6%) and gastrointestinal, paired with pulmonary complaints (21.6%). Purely gastrointestinal complaints led to the corresponding finding in 14.2% and a meconium ileus in 11.2%. In 6.7% of the cases, it was a sibling.
A general newborn screening has been carried out nationwide since September 1, 2016. It includes immunoreactive trypsin, pancreatitis-associated protein, and CFTR genetics.
therapy
Thanks to physiotherapy , inhalations and medication, especially the constantly improved digestive enzymes and antibiotics that have come onto the market in recent years, the prognosis of sick people has improved considerably in recent years. However, the treatment does not have a curative effect, but only symptomatically . Cystic fibrosis is a systemic defect that affects various organs. Since a causal treatment (e.g. via gene therapy ) is realistically impossible in around 97% of cases , each disorder of the individual organ systems must be treated separately.
Symptomatic treatment
Growth hormone therapy may be indicated in children who have delayed growth . Better growth with improved body weight also leads to fewer hospital admissions, fewer antibiotic treatments, and improved lung function . The supplementation with vitamins A , D , E and K are an important part of symptomatic treatment.
The patient's lungs are often affected by recurring infections that permanently damage the lung tissue. In particular, problem germs such as Pseudomonas aeruginosa , Burkholderia cepacia or resistant germs can cause severe pneumonia. Combating these germs is therefore of great importance. The lungs of most affected adults are chronically colonized with the bacterium Pseudomonas aeruginosa , which often leads to a deterioration in the lung situation. Some of the bacteria mentioned, e.g. B. Pseudomonas aeruginosa , together with the viscous mucus, form a biofilm in the patient's lungs. The tough mucus provides the bacteria with an ideal breeding ground in which they literally entrench themselves and are difficult to access for antibiotics. Here, high-dose antibiotics are usually administered intravenously and at three-month intervals over a period of 14 days.
In the antimicrobial treatment of cystic fibrosis, a distinction is made between four therapeutic principles:
- prophylactic long-term therapy (no pathogen detection, no symptoms)
- Early therapy or eradication treatment (pathogen detection, no symptoms)
- Exacerbation therapy (with or without evidence of pathogens, symptoms)
- Suppression therapy (chronic pathogen detection, no or chronic symptoms of the respiratory tract)
In addition to drugs for inhalation that loosen the thick mucus, inhalation drugs are also used to dilate the bronchi, as well as antibiotics and corticosteroids , which are also administered by inhalation. Ciprofloxacin - for children from 5 years - and gentamicin are usually administered against Pseudomonas aeruginosa . After inhaling expectorants, autogenous drainage or modified autogenous drainage is used. Both are specially developed breathing techniques that enable the patient to pump the viscous secretion up from the deep respiratory tract without outside help and then cough it up.
With increasing lung insufficiency, oxygen is permanently added to the breath ( oxygen long-term therapy ). Under the brand name Pulmozyme , recombinant human DNase (rhDNAse, Dornase alpha) is used as an inhaled drug to dissolve the DNA filaments present in the mucus. These DNA filaments are remnants of neutrophils . Neutrophil granulocytes are cells of the immune system that migrate into the lungs in order to attack bacteria that have settled there. The neutrophils are then disposed of by other cells of the immune system, said DNA filaments of the neutrophils remaining. These DNA filaments also contribute to the tenacity of the already tough mucus in the lungs. Administration of Dornase alpha reduces the spinnability of the mucus and improves mucociliary clearance.
In the case of special problems such as diabetes mellitus or impaired production of bile acids, these diseases must also be treated with medication. If an intestinal obstruction occurs, the so-called meconium ileus equivalent, medical help must be sought immediately.
There are as yet no guidelines for the treatment of osteoporosis in cystic fibrosis. The focus should be on preventive measures, including a healthy diet with calcium and vitamin D substitution and physical activity. In principle, existing osteoporosis can be treated with bisphosphonates , hormone replacement therapy or calcitonin . The few studies available on bisphosphonate treatment in patients with cystic fibrosis show an increase in bone density, but the number of bone fractures is not significantly reduced. No study results are yet available on the treatment of osteoporosis with raloxifene , strontium ranelate and teriparatide in patients with cystic fibrosis. The administration of growth hormones generally leads to increased bone density in children and adolescents.
Regular check-ups in a special outpatient department in the hospital, a university clinic or with a resident specialist are an essential part of the therapy.
In the spring of 2008, the indication for amitriptyline was extended to cystic fibrosis at the Essen Clinic . Amitriptyline indirectly inhibits acid sphingomyelinase and thus acts as FIASMA (functional inhibitor of acid sphingomyelinase).
Supportive measures
The weight loss caused by the exocrine pancreatic insufficiency is counteracted by the administration of high- energy, high-fat food and the administration of digestive enzymes ( pancreatins , fungal enzymes ). The body weight of cystic fibrosis patients is of great importance. The longer a normal or ideal weight can be maintained and underweight can be prevented, the more beneficial this is for lung function. Patients who are severely underweight usually show poorer lung function values during the check-ups than those with normal body weight or who are only minimally underweight. There are of course exceptions to this rule. It should be noted that the difficult breathing (e.g. due to obstruction of the lungs ) increases the energy expenditure again. This fact is usually taken into account by increasing the intake of food energy .
Supportive therapy includes regular physical activity such as running, jogging, cycling, dancing, etc. The most favorable sport for the individual is adapted to the respective state of health and recommended by the attending physician.
Lung transplant
Organ transplants , especially of the lungs, liver and pancreas, are now regularly carried out in transplant centers and represent an alternative in the treatment of cystic fibrosis for many people. The benefit of a lung transplant for this indication is, however, controversial.
If the one-second capacity FEV 1 of the lungs falls below a value of 30% of the normal range and coughing up blood ( hemoptysis ) occurs more frequently, the two-year survival rate is only around 50%. In such cases, a lung transplant may be appropriate. However, the waiting time for a donor organ is one to three years, and only every third to sixth patient can receive a donor organ. Because of the lack of organ donors , most of the applicants for a donor organ die. Usually a double lung transplant is performed. This is necessary because the immunosuppression used to prevent rejection of the donor organ would force infections of the remaining lungs. In addition, the donor organ would become infected. The three-year survival rate after a lung transplant is around 60%.
Drug treatment of the primary cause of disease
Drug treatment of the primary cause of the disease, i.e. the defect or the severely restricted function of the CFTR protein, is based on the six mutation classes. So-called correctors are being developed for mutation classes I and II, and potentiators for class III mutations . Correctors are designed to correct defective CFTR, and potentiators are designed to increase functionality or the number of chloride channels. The aim of the research is to increase the CFTR function to at least 5% of normal. It is believed that above this level, the severity of symptoms is significantly reduced or the main manifestations of the disease can be eliminated.
Approved drugs
With ivacaftor ( Kalydeco ) of Vertex Pharmaceuticals in 2012 the first drug was approved , which is directed against the primary cause of cystic fibrosis. Up to this point in time, cystic fibrosis could only be treated symptomatically. Ivacaftor was approved by the Food and Drug Administration (FDA) in January 2012 and by the European Medicines Agency (EMA) in July of that year for the treatment of patients over six years of age with a G551D mutation. About 4 to 5% of all cystic fibrosis patients have this mutation. This corresponds to about 3000 patients worldwide. In Europe, around 1500 cystic fibrosis patients have a G551D mutation. In Germany, only about 2% of cystic fibrosis patients have the necessary indication for treatment with ivacaftor. The era of personalized medicine for cystic fibrosis began with the approval of ivacaftor . Treatment costs in the United States are approximately $ 300,000 per patient per year. Ivacaftor was developed with financial support from the US patient organization Cystic Fibrosis Foundation . Vertex Pharmaceuticals received a total of $ 75 million. The Foundation receives a part of the profits from Vertex Pharmaceuticals. In Germany, the treatment costs of over € 330,000 per patient and year are fully covered by the statutory health insurance companies. In comparison, the drug costs of standard therapy are around € 21,000 per patient and year. A lung transplant is estimated at around € 150,000. The G551D mutation causes a class III defect in the CFTR channel. Ivacaftor belongs to the group of CFTR potentiators. It is a CFTR channel opener that opens the defective CFTR channel, thus increasing the decreased activity of CFTR. The first clinical results are promising. Patients gain weight rapidly and lung function improves significantly within a few weeks.
In February 2014, ivacaftor was approved by the FDA for the treatment of cystic fibrosis patients with eight other mutations. These are the mutations G178R, S549N, S549R, G551S, G1244E, S1251N, S1255P and G1349D.
A combination of ivacaftor and tezacaftor has now also been approved .
Experimental agents and treatment concepts
The discovery of the CFTR gene in 1989 opened up completely new avenues in the treatment of cystic fibrosis. High hopes were initially placed on gene therapy . The first gene therapy was carried out in 1993 in a cystic fibrosis patient. Over 20 further clinical studies followed. Various viral vectors were used for the transport of mRNA. So far, all clinical studies have been unsuccessful , despite promising preclinical data . The causes for the failure were varied and range from “insufficient efficiency of the transfection ” to “too short a duration of action” to “significant inflammatory side effects in the target tissue”.
The group of proofreaders in the mutation Class I heard the experimental drug ataluren . With Ataluren the correct reading of the CFTR gene should be made possible beyond an incorrectly set stop codon. Ataluren is said to be particularly effective with the stop codon UGA. Ataluren is available orally and initial clinical studies have shown promising results. Ataluren is currently (as of March 2015) in clinical phase III. The study is expected to end in November 2016.
Lumacaftor (code name VX-809) is an experimental agent that aims to eliminate CFTR defects in mutation class II. It is being clinically tested in combination with ivacaftor for the treatment of patients with the ΔF508 mutation. The rationale in combining a corrector with a potentiator is that the corrector enables the mutated CFTR protein to be transported to the cell membrane and that the potentiator should activate the function of the chloride channel there. The combination therapy Lumacaftor + Ivacaftor is currently (as of March 2015) in clinical phase III.
The group of proofreaders also includes chemical chaperones . These potential active ingredients should support the protein folding process or enable the correct protein folding of CFTR. They are therefore intended to compensate for class II genetic defects. Chemical chaperones have a non-specific effect on all cellular protein folding processes. One of these chaperones is sodium phenylbutyrate . Several clinical studies have been carried out with this active ingredient in the past. Although promising results were achieved, the doses required for this are extremely high, in the region of 20 grams per day, and the side effects are considerable, even with significantly lower doses. This therapeutic approach will therefore not be pursued any further. The last clinical study was canceled in 2011. Sodium phenylbutyrate is approved for the treatment of patients with hyperammonaemia . There is no approval for the treatment of cystic fibrosis. The potential active ingredient glycerol tri- (4-phenylbutyrate), a triglyceride of phenylbutyrate, is currently in clinical phase II for the treatment of cystic fibrosis. This compound is significantly more lipophilic and possibly better tolerated. The doses are also very high, in the range of 30 grams per day.
In June 2013 the EMA granted the furocoumarin 4,6,4′-trimethylangelicin (TMA) the status of an orphan drug . It is hoped that TMA will have a bifunctional mechanism of action. On the one hand, the compound is intended to restore the function of mutated CFTR or increase its activity and, on the other hand, have an anti-inflammatory effect by downregulating the expression of interleukin-8 . TMA is planned to be tested in clinical trials in patients with the ΔF508 mutation.
Model organisms
Suitable animal models that come as close as possible to human cystic fibrosis are required for research . This allows the physiological processes that lead to the pathological picture of the disease to be better understood and, above all, new active ingredients for treatment can be developed and pre-clinically tested before they are used in humans. The ideal animal model should reflect the essential characteristics such as inflammatory processes in the airways, the spontaneous development of bacterial infections and the progression to a chronic infection. In 1992, three years after the discovery of the CFTR gene, the CFTR -deficient mouse was developed. High hopes were placed in this model organism, but these could not be fulfilled. The "cystic fibrosis mouse" ( Cftr - knockout ), for example, does not develop any spontaneous infections in the lungs. Even the inoculation of large amounts of cystic fibrosis-typical lung pathogens are quickly eliminated by the immune system of the mice. For this reason the Cftr knockout mouse is not very suitable for the development of new antibacterial or anti-inflammatory treatment concepts.
Domestic pigs are more suitable . They have existed since 2008 as Cftr -deficient pigs and since 2011 also as homozygous ΔF508 pigs. These animals spontaneously develop the lung diseases typical of cystic fibrosis, which are characterized by infections, inflammations, heavy mucus and airway obstruction. The structure of the Airway Surface Liquid in humans and pigs are also similar. The cystic fibrosis ferret , which has been available since 2010, also develops spontaneous lung infections in early youth and is used as a model organism in cystic fibrosis research. Research on both animal models supports the hypothesis that the CFTR protein plays a direct role in mucosal immunity that goes far beyond the role of moistening the Airway Surface Liquid .
forecast
While many patients with cystic fibrosis died in adolescence two decades ago, there is now a good chance of reaching the age of 40 due to the continuously improving therapeutic options . For today's newborns, a value of 45 to 50 years is given. Worldwide, over 50% of cystic fibrosis patients are now over 18 years old.
Within the mutation classes, the prognosis generally becomes more favorable as the number increases. Classes I to III form a 'high risk group', and classes IV to VI form a 'low risk group'. In a study published in 2006, 1672 deaths in cystic fibrosis patients were assigned to these two risk groups. The patients in the low-risk group had a mean age of 37.6 ( IQR 28.8-47.9) and those in the high-risk group of 24.2 years (IQR 18.4-32.0). The minimally ill have a normal life expectancy and are able to father or carry children.
Due to the significantly improved prognosis, the clinical picture has also changed somewhat. Late complications such as osteoporosis and diabetes mellitus are now much more common. While cystic fibrosis used to be primarily a disease for paediatricians , it is now more and more important to internists and pulmonologists . In addition, there are also psychological side effects such as depression and anxiety , which are widespread in adult cystic fibrosis patients.
Heterozygous carriers of traits
Heterozygous CFTR mutation carriers cannot develop cystic fibrosis, but have a significantly lower expression of CFTR protein. A number of studies have looked at whether this might have other health effects - negative as well as positive.
General disease risks
It is certain that heterozygous CFTR mutation carriers are more susceptible to pancreatitis (inflammation of the pancreas). The risk of developing idiopathic chronic pancreatitis is around a factor of two to eleven times higher than in people without a defective CFTR gene. The exact reasons for this are still unknown.
As early as 1976, a study found that heterozygous CFTR mutation carriers are also more susceptible to allergies. The study results on the connection between an increased risk of asthma in heterozygous CFTR mutation carriers have been contradicting so far. They range from increased risk to a slight protective function.
Considered across the population as a whole, the reproductive capacity of female and male carriers of traits seems to correspond to that of the rest of the population. CFTR mutations also increase not mean that the risk of a chronic bronchitis , a chronic obstructive pulmonary disease developed (COPD).
Like cystic fibrosis patients, carriers of traits have reduced blood pressure . In the past, this finding was explained by the increased loss of electrolytes . However, more recent studies from 2013 show that the reduced CFTR expression also causes changes in the blood vessels. The agonist- induced release of calcium ions by the smooth muscle cells of the aorta is reduced. This also reduces blood pressure. The effect of reduced blood pressure on carriers of traits is particularly noticeable in advanced age and in systolic blood pressure. In a British study with more than 1200 test persons, the systolic blood pressure of heterozygous women was 7 mmHg and the diastolic blood pressure 4 mmHg lower than in the comparison group. Decreased blood pressure provides increased protection against stroke and coronary artery disease . From the blood pressure values, the authors of the study calculated a 30% reduction in the risk of stroke and a 20% reduction in the risk of myocardial infarction for heterozygous women .
Studies have shown that CFTR heterozygous women do not have reduced fertility compared to women with two non-mutated CFTR alleles.
Selection advantage of the heterozygous genotype
There is currently no definitive explanation for why an allele that causes such a serious disease is so widespread and not in the course of evolution selected out was. In small, isolated populations, the frequency of cystic fibrosis can be explained by genetic drift and the founder effect . In general, a fatal recessive hereditary disease in large populations cannot achieve such a high frequency through these two effects alone. All population genetic data suggest a heterozygous advantage as the main cause of the high frequency of the most important CFTR mutations. This means that the loss of function in only one of the two CFTR alleles, which leads to a reduction in the number of functional chloride channels, results in a selection advantage. The best known example of a heterozygous advantage are heterozygous carriers of traits in sickle cell anemia . They are largely symptom-free, but are significantly less likely to develop malaria. In the case of cystic fibrosis, a number of hypotheses have been drawn up as to which diseases the heterozygous carriers of the trait show an increased resistance. This resistance offers the selection advantage, which in turn has led to the extremely frequent spread of the gene defect. To this day, this selection advantage, which has made cystic fibrosis one of the most common hereditary diseases, has not been determined with certainty.
Among other things, higher resistance to certain pathogens is suspected . With the discovery that the cholera toxin leads to increased CFTR expression in the intestinal epithelia, which leads to massive water loss in cholera , the hypothesis was put forward that this is the selection advantage for heterozygous carriers of traits. The bacterium Salmonella Typhimurium reaches the epithelia via CFTR, which is why it is hypothesized that the reduced CFTR expression in heterozygous carriers reduces the likelihood of typhoid fever . This pathogen also promotes the expression of CFTR. In areas where typhus is endemic, the correlation between the likelihood of illness and the CFTR genotype was confirmed. However, cystic fibrosis is extremely rare in the areas investigated, and no ΔF508 mutation could be found in any of the 775 test subjects. The areas of spread of cholera and typhoid do not correlate with the frequency of the CFTR mutation in these areas, which is an indication against these two hypotheses. Mathematical models based on historical demographic and epidemiological data also show that neither cholera nor typhus could have sufficient selection pressure to explain the high incidence of cystic fibrosis.
In contrast, these models, together with clinical and molecular biological data, suggest that the selection pressure in tuberculosis was sufficiently high in the past. From the beginning of the 16th century to the beginning of the 20th century, tuberculosis was pandemic (“white plague”) in Europe and responsible for over 20% of deaths. Tuberculosis therefore has an extremely high selection pressure. The tuberculosis hypothesis was put forward as early as 1967 on the basis of the clinical observation that cystic fibrosis patients rarely develop tuberculosis. Later, a lower mortality from tuberculosis was found in heterozygous CFTR carriers. The cause of the increased resistance of cystic fibrosis patients against tuberculosis is probably the reduced activity of the enzyme arylsulfatase B . The disease-causing mycobacteria have the enzyme arylsulfotransferase instead of arylsulphatase B , which is why they are dependent on sulphate sources from their host to build up their cell wall . If these sources are missing, the mycobacteria cannot multiply sufficiently. The tuberculosis hypothesis fulfills the three criteria for selection factors: the molecular-biological, the clinical and the historical-geographical criterion. The first appearance of Mycobacterium tuberculosis about 35,000 years ago, which corresponds to the origin of cystic fibrosis, also speaks in favor of the tuberculosis hypothesis . Assuming that this tuberculosis hypothesis is correct, the incidence of cystic fibrosis should fall by 0.1% per year over the next 100 years with the significantly lower tuberculosis mortality of people of childbearing age in developed countries. It would take around 20 generations in these countries to halve the incidence.
Another hypothesis is based on a correlation between general adult lactose tolerance and the incidence of disease in certain colonies. The rate is highest in the European and North American populations of European descent, each with a high lactose tolerance, while it is lowest in Asia for the widespread lactose intolerance there. From this a connection could be derived and also a selection advantage for heterozygous trait carriers who have not yet let the gene defect become extinct.
Hypotheses about an increased fertility of heterozygous trait carriers as a selection advantage could not be confirmed epidemiologically.
Medical history
From mutation frequency analyzes we know that cystic fibrosis is a very old gene mutation. The most common types of mutation in the CFTR gene, such as ΔF508, originated around 51,000 years ago in the Arab-Middle East region. The ethnic group of the Baluch was probably the original population. At that time they lived on the Persian Plateau in a central location on a cross- peoples migration route . This way, the cystic fibrosis was able to spread quickly to Europe by hunters. It appeared there before the last Ice Age , around 30,000 to 40,000 BC. At the beginning of the Upper Palaeolithic , first on.
Looking back, the clinical picture of cystic fibrosis can be found in a number of case studies from the mid-17th century in the medical literature. However, there was no recognition that this was an independent multi-system disease. The first detailed description of the symptoms of a swollen, hardened, whitish shimmering pancreas comes from an autopsy report by the Leiden anatomist Peter Pauw from the year 1595. Pauw examined the corpse of an allegedly bewitched 11-year-old girl.
Centuries ago, the salty taste of babies was recognized as an ominous sign of child health and shortened life expectancy. Ernst Ludwig Rochholz wrote about this in 1857 in his book Alemannic children's song and children's play from Switzerland :
"The child will soon die again whose forehead tastes salty when kissed."
In the literature, the version from a dictionary of the Swiss German language is often quoted:
"Woe to the child who tastes salty when kissed on the forehead, it is bewitched and will soon die."
In 1936, the Swiss pediatrician Guido Fanconi first described the clinical picture of cystic fibrosis as “celiac syndrome in congenital cystic pancreatic fibromatosis” . In the publication, Fanconi and his two co-authors describe two cases of an apparently fatal illness in young children. At the time, they assumed that it was a very rare syndrome.
The Austrian Karl Landsteiner , the discoverer of the blood groups, had already described the symptoms of the disease in 1905. In it, Landsteiner describes the case of a girl who died on the fifth day of her life “with a distended stomach”. During the autopsy, he found that the meconium was gray-yellow and of an extremely tough consistency, like “thickened glass putty”. In this state it could not be moved by the forces of the intestine. Landsteiner stated:
"It can therefore be seen that the abnormal nature of the meconium was the ultimate cause of the intestinal obstruction, since the thickening has existed for a long time (ie already in the womb. FK)"
In the girl's pancreas, he found a "very significant increase" in connective tissue (fibrosis).
The term "Cystic Fibrosis" (Engl. Cystic fibrosis ) shaped the American pediatrician and pathologist Dorothy Hansine Andersen 1938. They, turning toward the tissue changes of the affected organs with mucous glands. In addition, she was the first to define the clinical picture. The American pathologist Sidney Farber called the disease "cystic fibrosis" in 1944 because of the production of thick mucus. This designation has established itself especially in German-speaking countries.
With the availability of the antibiotics penicillin (from 1944), chlortetracycline (from 1948), oxytetracycline (from 1950), chloramphenicol and erythromycin (both from 1951), the basis of palliative therapy for cystic fibrosis was created.
In 1949, Charles Upton Lowe (1921–2012) recognized that cystic fibrosis is a hereditary disease. He also found autosomal recessive inheritance and postulated that the disease is caused by a defect in a single gene. The first publication about the increased electrolyte content in the sweat of cystic fibrosis patients comes from the year 1953 by Paul di Sant'Agnese (1914-2005) and colleagues. They found that nine children with cystic fibrosis had a three-fold increase in the chloride ion concentration in their sweat. This knowledge is still used today in the sweat test for diagnosis. In addition, a scientific basis was created for the knowledge from the Middle Ages about the salty and bitter taste of sweat in children with cystic fibrosis. This knowledge was also the basis for the development of the pilocarpine iontophoresis sweat test by Lewis E. Gibson (1927-2008) and Robert E. Cooke (1920-2014) in 1959. Paul M. Quinton found the cause of the increased salt concentration 1983 when he examined isolated sweat gland ducts ( ductus sudoriferus ) of cystic fibrosis patients and found very low sodium chloride reabsorption, which is caused by an abnormally low chloride ion permeability of the endothelia. Two years later, the molecular genetic basis of cystic fibrosis was found. An international working group headed by Robert G. Knowlton identified chromosome 7 as the location of the genetic defect. This was done via linkage analyzes in families with children with cystic fibrosis. In 1989, the CFTR gene was cloned for the first time and the three-base deletion ΔF508 was recognized as the mutation responsible for most cases of cystic fibrosis. An important aid in the search for the CFTR gene was the three-base deletion, which was present in about 70% of the cystic fibrosis patients examined at the time. At the time of cloning it was not yet known whether the CFTR gene codes for the chloride channel or for a regulator of a chloride channel. That is why the name cystic fibrosis transmembrane conductance regulator was chosen to cover both possibilities.
As a result of the growing knowledge about the pathophysiology of cystic fibrosis, new therapy concepts could be developed in the period that followed, with which the life expectancy of those affected could be increased considerably. The preliminary highlight is the approval of the first drug, which was granted in 2012, with which the cause of cystic fibrosis can be treated in some patients.
See also
- Lubani-al-Saleh-Teebi syndrome (cystic fibrosis with gastritis and megaloblastic anemia)
literature
Reference books
- Dietrich Reinhardt, Manfred Götz, Richard Kraemer, Martin H. Schöni (eds.): Cystic fibrosis. Springer-Verlag, 2013, ISBN 978-3-642-56796-4 , 611 pages ( limited preview in the Google book search).
- Hermann Lindemann, Burckhardt Tümmler, Gerhard Dockter (eds.): Mukoviszidose - cystic fibrosis. 4th edition, Georg Thieme, 2004, ISBN 3-13-138604-5 , 174 pp. ( Limited preview in Google book search)
- Tom O. Hirche, Thomas OF Wagner: Update cystic fibrosis. Georg Thieme Verlag, 2013, ISBN 978-3-13-176981-7 , 136 p. ( Limited preview in Google book search)
- Margaret Hodson, Andrew Bush, Duncan Geddes: Cystic Fibrosis. 3rd edition, CRC Press, 2012, ISBN 978-1-4441-1369-3 , 486 pp. ( Limited preview in Google book search)
- German Society for Pediatric Infectious Diseases (DGPI): DGPI manual. Infections in children and adolescents. ZDB ID 1308754-x .
- Roland Busch: History about cystic fibrosis. Hanover 1995.
Trade journals
- Journal of Cystic Fibrosis official peer-reviewed journal of the European Cystic Fibrosis Society (ECFS)
Guidelines
- S1 guideline for cystic fibrosis: Nutrition and exocrine pancreatic insufficiency of the Society for Pediatric Gastroenterology and Nutrition (GPGE). In: AWMF online (as of 2011)
- S1 guideline diagnosis of cystic fibrosis of the German Society for Child and Adolescent Medicine (DGKJ). In: AWMF online (as of 2013)
Web links
- Cystic fibrosis. In: Online Mendelian Inheritance in Man . (English)
- Cystic fibrosis. In: Orphanet (Rare Disease Database).
- Federal Association of Cystic Fibrosis (CF) Mukovisdose e. V.
- Cystic fibrosis - learning program for medical students at the University of Bern
- Lungeninformationsdienst.de - cystic fibrosis
- Mukoland.de - long-term blog of a lung transplanted cystic fibrosis patient
Individual evidence
- ↑ a b c d Cystic Fibrosis Mutation Database: Statistics. (No longer available online.) In: genet.sickkids.on.ca. Archived from the original on July 11, 2017 ; accessed on June 27, 2017 (English). Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b M. S. Gelman, RR Kopito: Cystic fibrosis: premature degradation of mutant proteins as a molecular disease mechanism. In: Methods in molecular biology. Volume 232, 2003, pp. 27-37, doi: 10.1385 / 1-59259-394-1: 27 , PMID 12840537 (review).
- ^ A. Jaffé, A. Bush: Cystic fibrosis: review of the decade. In: Monaldi archives for chest disease. Volume 56, Number 3, June 2001, pp. 240-247, PMID 11665504 (review).
- ↑ a b Alexander Knorre: Investigation of the ER overload response mediated by the transcription factor NF-κB in cystic fibrosis. Dissertation, Albert-Ludwigs-University Freiburg im Breisgau, 2001, p. 30.
- ^ A b c Daniel Merk, Manfred Schubert-Zsilavecz: Personalized medicine: New approaches in cystic fibrosis. In: pharmische-zeitung.de. 2011, accessed March 13, 2015 .
- ^ SC Bell, K. De Boeck, MD Amaral: New pharmacological approaches for cystic fibrosis: promises, progress, pitfalls. In: Pharmacology & therapeutics. Volume 145, January 2015, pp. 19-34, doi: 10.1016 / j.pharmthera.2014.06.005 , PMID 24932877 .
- ↑ Answer of the Federal Government to a small question : Combating cystic fibrosis in childhood BT-Drs. 9/2188 of November 26, 1982 (PDF file)
- ↑ a b c d e f g Sabina Gallati: Function of CFTR as a chloride channel on the plasma membrane. In: Dietrich Reinhardt, Manfred Götz, Richard Kraemer, Martin H. Schöni (Eds.): Cystic Fibrosis. Springer-Verlag, 2013, ISBN 978-3-642-56796-4 , pp. 1–19 ( limited preview in Google book search).
- ↑ a b M. Hergersberg, J. Balakrishnan u. a .: A new mutation, 3905insT, accounts for 4.8% of 1173 CF chromosomes in Switzerland and causes a severe phenotype. In: Human genetics. Volume 100, Number 2, August 1997, pp. 220-223, PMID 9254853 .
- ↑ [1]
- ^ G. Romeo, M. Devoto, LJ Galietta: Why is the cystic fibrosis gene so frequent? In: Human genetics. Volume 84, Number 1, December 1989, pp. 1-5, PMID 2691388 (review).
- ↑ a b G. Lucotte, S. Hazout, M. De Braekeleer: Complete map of cystic fibrosis mutation DF508 frequencies in Western Europe and correlation between mutation frequencies and incidence of disease. In: Human biology. Volume 67, Number 5, October 1995, pp. 797-803, PMID 8543293 .
- ↑ a b c d J. L. Bobadilla, M. Macek u. a .: Cystic fibrosis: a worldwide analysis of CFTR mutations – correlation with incidence data and application to screening. In: Human mutation. Volume 19, Number 6, June 2002, pp. 575-606, doi: 10.1002 / humu.10041 , PMID 12007216 (review).
- ↑ Y. Yamashiro, T. Shimizu, et al. a .: The estimated incidence of cystic fibrosis in Japan. In: Journal of pediatric gastroenterology and nutrition. Volume 24, Number 5, May 1997, pp. 544-547, PMID 9161949 .
- ↑ a b C. Castellani, H. Cuppens a. a .: Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. In: Journal of cystic fibrosis. Volume 7, number 3, May 2008, pp. 179-196, doi: 10.1016 / j.jcf.2008.03.009 , PMID 18456578 , PMC 2810954 (free full text) (review).
- ↑ a b N. Morral, J. Bertranpetit u. a .: The origin of the major cystic fibrosis mutation (delta F508) in European populations. In: Nature genetics. Volume 7, Number 2, June 1994, pp. 169-175, doi: 10.1038 / ng0694-169 , PMID 7920636 .
- ↑ X. Estivill, C. Bancells, C. Ramos: Geographic distribution and regional origin of 272 cystic fibrosis mutations in European populations. The Biomed CF Mutation Analysis Consortium. In: Human mutation. Volume 10, Number 2, 1997, pp. 135-154, doi : 10.1002 / (SICI) 1098-1004 (1997) 10: 2 <135 :: AID-HUMU6> 3.0.CO; 2-J , PMID 9259197 .
- ↑ O. Lao, AM Andrés u. a .: Spatial patterns of cystic fibrosis mutation spectra in European populations. In: European journal of human genetics. Volume 11, Number 5, May 2003, pp. 385-394, doi: 10.1038 / sj.ejhg.5200970 , PMID 12734544 .
- ↑ E. Mateu, F. Calafell et al. a .: Can a place of origin of the main cystic fibrosis mutations be identified? In: American Journal of Human Genetics . Volume 70, number 1, January 2002, pp. 257-264, doi: 10.1086 / 338243 , PMID 11713719 , PMC 384895 (free full text).
- ↑ a b J. Sanz, T. von Känel u. a .: The CFTR frameshift mutation 3905 insT and its effect at transcript and protein level. In: European journal of human genetics. Volume 18, number 2, February 2010, pp. 212-217, doi: 10.1038 / ejhg.2009.140 , PMID 19724303 , PMC 2987192 (free full text).
- ↑ a b Sabina Gallati, Dominik Hartl u. a .: Cystic fibrosis. In: Erika von Mutius, Monika Gappa u. a .: Pediatric pulmonology. Springer-Verlag, 2013, ISBN 3-642-34827-0 , pp. 587-632 ( limited preview in Google book search)
- ↑ a b c d e Thilo Dörk, Manfred Stuhrmann: Mukoviszidose (cystic fibrosis, CF). In: Detlev Ganten , Klaus Ruckpaul : Monogenic Hereditary Diseases Volume 6, Springer-Verlag, 2013, ISBN 3-642-57043-7 , pp. 173-194, limited preview in the Google book search
- ^ The CFTR mutations database: The CFTR gene. In: Universal Mutation Database . Retrieved March 2, 2015 .
- ↑ R. Sebro, H. Levy et al. a .: Cystic fibrosis mutations for p.F508del compound heterozygous predict sweat chloride levels and pancreatic sufficiency. In: Clinical genetics. Volume 82, number 6, December 2012, pp. 546-551, doi: 10.1111 / j.1399-0004.2011.01804.x , PMID 22035343 , PMC 4279028 (free full text).
- ↑ a b A. Ahmad, A. Ahmed, P. Patrizio: Cystic fibrosis and fertility. In: Current opinion in obstetrics & gynecology. Volume 25, Number 3, June 2013, pp. 167-172, doi: 10.1097 / GCO.0b013e32835f1745 , PMID 23429570 (review).
- ^ MP Rogan, DA Stoltz, DB Hornick: Cystic fibrosis transmembrane conductance regulator intracellular processing, trafficking, and opportunities for mutation-specific treatment. In: Chest. Volume 139, Number 6, June 2011, pp. 1480-1490, doi: 10.1378 / chest.10-2077 , PMID 21652558 (review).
- ^ A b C. L. Ward, RR Kopito: Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. In: The Journal of biological chemistry. Volume 269, Number 41, October 1994, pp. 25710-25718, PMID 7523390 .
- ^ MD Amaral: CFTR and chaperones: processing and degradation. In: Journal of molecular neuroscience. Volume 23, number 1-2, 2004, pp. 41-48, doi: 10.1385 / JMN: 23: 1-2: 041 , PMID 15126691 (review).
- ^ A b S. J. Kim, WR Skach: Mechanisms of CFTR Folding at the Endoplasmic Reticulum. In: Frontiers in pharmacology. Volume 3, 2012, p. 201, doi: 10.3389 / fphar.2012.00201 , PMID 23248597 , PMC 3521238 (free full text).
- ^ DN Hebert, M. Molinari: In and out of the ER: protein folding, quality control, degradation, and related human diseases. In: Physiological reviews. Volume 87, Number 4, October 2007, pp. 1377-1408, doi: 10.1152 / physrev.00050.2006 , PMID 17928587 (review).
- ↑ CL Ward, S. Omura, RR Kopito: Degradation of CFTR by the ubiquitin-proteasome pathway. In: Cell. Volume 83, Number 1, October 1995, pp. 121-127, PMID 7553863 .
- ↑ a b J. R. Riordan, JM Rommens u. a .: Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. In: Science. Volume 245, Number 4922, September 1989, pp. 1066-1073, PMID 2475911 .
- ^ DN Sheppard, MJ Welsh: Structure and function of the CFTR chloride channel. In: Physiological reviews. Volume 79, Number 1 Suppl, January 1999, pp. S23-S45, PMID 9922375 (review).
- ↑ a b V. Im Hof, P. Gehr: Mucociliary clearance. In: Christian Rieger, Horst von der Hardt u. a. (Ed.): Pediatric Pneumology. 2nd edition, Springer-Verlag, 2013, ISBN 3-662-09182-8 , pp. 110–117 ( limited preview in the Google book search)
- ↑ JM Pilewski, RA Frizzell: Role of CFTR in airway disease. In: Physiological reviews. Volume 79, Number 1 Suppl, January 1999, pp. S215-S255, PMID 9922383 (review).
- ^ Albrecht Schwab: Breathing and Acid-Base Balance. In: Michael Gekle u. a. (Ed.): Pocket textbook Physiology. Thieme Verlag, Stuttgart 2010, ISBN 978-3-13-144981-8 , p. 281.
- ↑ a b J. Zabner, JJ Smith u. a .: Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. In: Molecular cell. Volume 2, Number 3, September 1998, pp. 397-403, PMID 9774978 .
- ↑ a b J. J. Smith, SM Travis et al. a .: Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. In: Cell. Volume 85, Number 2, April 1996, pp. 229-236, PMID 8612275 .
- ↑ H. Matsui, BR Grubb et al. a .: Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. In: Cell. Volume 95, Number 7, December 1998, pp. 1005-1015, PMID 9875854 .
- ↑ Geraldine Leier: Mechanism of CFTR activation by Sildenafil with regard to cystic fibrosis. Dissertation, Westfälische Wilhelms-Universität Münster, 2011, p. 6.
- ↑ R. Tarran, BR Grubb et al. a .: The CF salt controversy: in vivo observations and therapeutic approaches. In: Molecular cell. Volume 8, Number 1, July 2001, pp. 149-158, PMID 11511368 .
- ↑ WB Guggino: Cystic fibrosis and the salt controversy. In: Cell. Volume 96, Number 5, March 1999, pp. 607-610, PMID 10089875 (review).
- ↑ WB Guggino: Cystic fibrosis salt / fluid controversy: in the thick of it. In: Nature medicine. Volume 7, Number 8, August 2001, pp. 888-889, doi: 10.1038 / 90914 , PMID 11479614 .
- ↑ ME Krouse: Is cystic fibrosis lung disease Caused by abnormal ion composition or abnormal volume? In: The Journal of general physiology. Volume 118, Number 2, August 2001, pp. 219-222, PMID 11479348 , PMC 2233826 (free full text) (review).
- ↑ Andrew Bush: Cystic Fibrosis. In: John A. Goodfellow (Ed.): Understanding Medical Research: The Studies That Shaped Medicine. John Wiley & Sons, 2012, ISBN 1-119-96373-7 , p. 88 limited preview in the Google book search
- ↑ a b C. Randak, Burckhardt Tümmler: Function of CFTR as a chloride channel on the plasma membrane. In: Dietrich Reinhardt, Manfred Götz, Richard Kraemer, Martin H. Schöni (Eds.): Cystic Fibrosis. Springer-Verlag, 2013, ISBN 978-3-642-56796-4 , pp. 29–33 ( limited preview in Google book search).
- ↑ K. Ho: The ROMK-cystic fibrosis transmembrane conductance regulator connection: new insights into the relationship between ROMK and cystic fibrosis transmembrane conductance regulator channels. In: Current opinion in nephrology and hypertension. Volume 7, Number 1, January 1998, pp. 49-58, PMID 9442363 (review).
- ↑ LC Boujaoude, C. Bradshaw-Wilder and a .: Cystic fibrosis transmembrane regulator regulates uptake of sphingoid base phosphates and lysophosphatidic acid: modulation of cellular activity of sphingosine 1-phosphate. In: The Journal of biological chemistry. Volume 276, Number 38, September 2001, pp. 35258-35264, doi: 10.1074 / jbc.M105442200 , PMID 11443135 .
- ^ A b S. M. Rowe, S. Miller, EJ Sorscher: Cystic fibrosis. In: The New England Journal of Medicine . Volume 352, Number 19, May 2005, pp. 1992-2001, doi: 10.1056 / NEJMra043184 , PMID 15888700 .
- ↑ EF McKone, SS Emerson u. a .: Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. In: The Lancet. Volume 361, Number 9370, May 2003, pp. 1671-1676, doi: 10.1016 / S0140-6736 (03) 13368-5 , PMID 12767731 .
- ↑ a b E. F. McKone, CH Goss, ML Aitken: CFTR genotype as a predictor of prognosis in cystic fibrosis. In: Chest. Volume 130, Number 5, November 2006, pp. 1441-1447, doi: 10.1378 / chest.130.5.1441 , PMID 17099022 .
- ^ EW Alton: A mild variant of cystic fibrosis. In: Thorax. Volume 51 Suppl 2, August 1996, pp. S51-S54, PMID 8869353 , PMC 1090707 (free full text) (review).
- ↑ L. Cebotaru, D. Rapino et al. a .: Correcting the cystic fibrosis disease mutant, A455E CFTR. In: PloS one. Volume 9, number 1, 2014, p. E85183, doi: 10.1371 / journal.pone.0085183 , PMID 24416359 , PMC 3885674 (free full text).
- ↑ a b c d e f M. P. Boyle, K. De Boeck: A new era in the treatment of cystic fibrosis: correction of the underlying CFTR defect. In: The Lancet. Respiratory medicine. Volume 1, Number 2, April 2013, pp. 158-163, doi: 10.1016 / S2213-2600 (12) 70057-7 , PMID 24429096 (review).
- ^ E. Kerem: Pharmacologic therapy for stop mutations: how much CFTR activity is enough? In: Current opinion in pulmonary medicine. Volume 10, Number 6, November 2004, pp. 547-552, PMID 15510065 (review).
- ↑ a b c Ernst Joachim Rietschel: Cystic fibrosis: From symptomatic to complementary mutation-specific therapy. ( Memento of the original from April 2, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Habilitation thesis, University of Cologne, 2013, p. 4.
- ↑ M. Haardt, M. Benharouga et al. a .: C-terminal truncations destabilize the cystic fibrosis transmembrane conductance regulator without impairing its biogenesis. A novel class of mutation. In: The Journal of biological chemistry. Volume 274, Number 31, July 1999, pp. 21873-21877, PMID 10419506 .
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , pp. 193–197, here: p. 193.
- ↑ Marianne Abele-Horn (2009), p. 195.
- ↑ Marianne Abele-Horn (2009), p. 193 and (on antimicrobial therapy) p. 196.
- ↑ Dirk Stolz: On the frequency of the mutation CFTRdele2,3 (21kb) in the CFTR gene in a German patient collective. Dissertation, Justus Liebig University Giessen, 2006, p. 2.
- ↑ AM Cantin, G. Bilodeau et al. a .: Oxidant stress suppresses CFTR expression. In: American journal of physiology. Cell physiology. Volume 290, Number 1, January 2006, pp. C262-C270, doi: 10.1152 / ajpcell.00070.2005 , PMID 16162662 .
- ↑ AM Cantin, JW Hanrahan et al. a .: Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. In: American journal of respiratory and critical care medicine. Volume 173, Number 10, May 2006, pp. 1139-1144, doi: 10.1164 / rccm.200508-1330OC , PMID 16497995 .
- ↑ EF McKone, J. Shao et al. a .: Variants in the glutamate-cysteine-ligase gene are associated with cystic fibrosis lung disease. In: American journal of respiratory and critical care medicine. Volume 174, number 4, August 2006, pp. 415-419, doi: 10.1164 / rccm.200508-1281OC , PMID 16690975 , PMC 2648118 (free full text).
- ↑ a b T. O. Hirche, TOF Wagner: Mukoviszidose (cystic fibrosis). In: Heinrich Matthys, Werner Seeger: Clinical Pneumology. 4th edition, Springer Science & Business Media, 2008, ISBN 3-540-37682-8 , pp. 280-295, limited preview in the Google book search
- ↑ J. Hein: Mukoviszidose (cystic fibrosis). In: Nikolaus Konietzko, H. Wendel, B. Wiesner: Diseases of the lungs. Walter de Gruyter, 1994 ISBN 3-11-012130-1 , p. 619 ( limited preview in the Google book search)
- ↑ a b M. Ballmann: Mukoviszidose und Diabetes. In: The Diabetologist. Volume 6, number 1, 2010, pp. 16–22 doi: 10.1007 / s11428-009-0437-6 (see also insulin therapy for type 3 diabetes extended lifespan ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. )
- ↑ J. Herwig, H. Böhles: Diabete mellitus in children and adolescents. In: Hellmut Mehnert, Eberhard Standl u. a. (Ed.): Diabetology in clinic and practice. 5th edition, Georg Thieme Verlag, 2003, ISBN 3-13-512805-9 , p. 341 ( limited preview in the Google book search)
- ↑ T. Flass, MR Narkewicz: Cirrhosis and other liver disease in cystic fibrosis. In: Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. Volume 12, number 2, March 2013, pp. 116-124, doi: 10.1016 / j.jcf.2012.11.010 , PMID 23266093 , PMC 3883947 (free full text).
- ↑ M. Angelico, C. Gandin et al. a .: Gallstones in cystic fibrosis: a critical reappraisal. In: Hepatology (Baltimore, Md.). Volume 14, Number 5, November 1991, pp. 768-775, PMID 1937382 .
- ^ A b N. C. Dean, DH Van Boerum, TG Liou: Rib plating of acute and sub-acute non-union rib fractures in an adult with cystic fibrosis: a case report. In: BMC research notes. Volume 7, 2014, p. 681, doi: 10.1186 / 1756-0500-7-681 , PMID 25270323 , PMC 4197343 (free full text).
- ↑ a b c d e f g R. M. Javier, J. Jacquot: Bone disease in cystic fibrosis: what's new? In: Joint, bone, spine. Volume 78, Number 5, October 2011, pp. 445-450, doi: 10.1016 / j.jbspin.2010.11.015 , PMID 21233000 (review).
- ^ A b L. S. Conwell, AB Chang: Bisphosphonates for osteoporosis in people with cystic fibrosis. In: The Cochrane database of systematic reviews. Volume 3, 2014, S. CD002010, doi: 10.1002 / 14651858.CD002010.pub4 , PMID 24627308 (Review).
- ^ G. Döring, SP Conway: Osteoporosis in cystic fibrosis. In: Jornal de pediatria. Volume 84, Number 1, 2008, pp. 1-3, doi: 10.2223 / JPED.1749 , PMID 18264617 .
- ↑ CS Haworth, PL Selby et al. a .: Osteoporosis in adults with cystic fibrosis. In: Journal of the Royal Society of Medicine. Volume 91 Suppl 34, 1998, pp. 14-18, PMID 9709383 , PMC 1296368 (free full text) (review).
- ↑ a b R. M. Aris, JB Renner u. a .: Increased rate of fractures and severe kyphosis: sequelae of living into adulthood with cystic fibrosis. In: Annals of internal medicine. Volume 128, Number 3, February 1998, pp. 186-193, PMID 9454526 .
- ↑ SM Lang, R. Fischer u. a .: High prevalence of osteoporosis in adult patients with cystic fibrosis. In: German Medical Weekly (1946). Volume 129, Numbers 28-29, July 2004, pp. 1551-1555, doi: 10.1055 / s-2004-828988 , PMID 15243902 .
- ↑ EF Shead, CS Haworth et al. a .: Osteoclast function, bone turnover and inflammatory cytokines during infective exacerbations of cystic fibrosis. In: Journal of cystic fibrosis. Volume 9, Number 2, March 2010, pp. 93-98, doi: 10.1016 / j.jcf.2009.11.007 , PMID 20006563 .
- ↑ EF Shead, CS Haworth et al. a .: Osteoclastogenesis during infective exacerbations in patients with cystic fibrosis. In: American journal of respiratory and critical care medicine. Volume 174, Number 3, August 2006, pp. 306-311, doi: 10.1164 / rccm.200512-1943OC , PMID 16675777 .
- ↑ EF Shead, CS Haworth et al. a .: Cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in human bone. In: Thorax. Volume 62, number 7, July 2007, pp. 650-651, doi: 10.1136 / thx.2006.075887 , PMID 17600296 , PMC 2117234 (free full text).
- ↑ L. Le Heron, C. Guillaume et al. a .: Cystic fibrosis transmembrane conductance regulator (CFTR) regulates the production of osteoprotegerin (OPG) and prostaglandin (PG) E2 in human bone. In: Journal of cystic fibrosis. Volume 9, Number 1, January 2010, pp. 69-72, doi: 10.1016 / j.jcf.2009.11.005 , PMID 20005786 .
- ↑ E. Shane, SJ Silverberg et al. a .: Osteoporosis in lung transplantation candidates with end-stage pulmonary disease. In: The American journal of medicine. Volume 101, Number 3, September 1996, pp. 262-269, doi: 10.1016 / S0002-9343 (96) 00155-6 , PMID 8873487 .
- ↑ RZ Sokol: Infertility in men with cystic fibrosis. In: Current opinion in pulmonary medicine. Volume 7, Number 6, November 2001, pp. 421-426, PMID 11706320 (review).
- ↑ W. Lissens, I. Liebaers: The genetics of male infertility in relation to cystic fibrosis. In: Baillière's clinical obstetrics and gynecology. Volume 11, Number 4, December 1997, pp. 797-817, PMID 9692018 (review).
- ↑ J. Yu, Z. Chen et al. a .: CFTR mutations in men with congenital bilateral absence of the vas deferens (CBAVD): a systemic review and meta-analysis. In: Human reproduction. Volume 27, Number 1, January 2012, pp. 25-35, doi: 10.1093 / humrep / der377 , PMID 22081250 (review).
- ^ A b c d Gerhard Dockter, Hermann Lindemann: Genetics, molecular biology and general pathophysiology. In: Hermann Lindemann, Burckhardt Tümmler, Gerhard Dockter (ed.): Mukoviszidose - Cystic Fibrosis. 4th edition, Georg Thieme, 2004, ISBN 3-13-138604-5 , pp. 3–13, limited preview in Google book search
- ^ Olaf Sommerburg: Cystic fibrosis: genetic defect with dire consequences. In: pharmische-zeitung.de. 2008, accessed March 16, 2015 .
- ^ A b Claus Kroegel, Ulrich Costabel: Clinical Pneumology: The reference work for clinic and practice. Georg Thieme, 2013, ISBN 3-13-175781-7 , p. 655 limited preview in the Google book search
- ↑ MJ Müller, J. Westenhöfer u. a .: Nutritional medical examinations. In: Manfred James Müller: Nutritional Medicine Practice: Methods - Prevention - Treatment. 2nd edition, Springer-Verlag, 2007, ISBN 3-540-38231-3 , p. 162 limited preview in the Google book search
- ^ J. Massie, B. Clements: Diagnosis of cystic fibrosis after newborn screening: the Australasian experience - twenty years and five million babies later: a consensus statement from the Australasian Pediatric Respiratory Group. In: Pediatric pulmonology. Volume 39, number 5, May 2005, pp. 440-446, doi: 10.1002 / ppul.20191 , PMID 15704202 (review).
- ↑ Corina S. Rueegg, Claudia E. Kuehni, Sabina Gallati, Matthias Baumgartner, Toni torresani, Juerg Barben: neonatal screening for cystic fibrosis in Switzerland: Evaluation after one year . In: Deutsches Ärzteblatt . tape 110 , no. 20 . Deutscher Ärzte-Verlag , May 17, 2013, p. 356–63 , doi : 10.3238 / arztebl.2013.0356 .
- ↑ Cystic Fibrosis: Newborn Screening in the Northeast. In: aerztezeitung.de. October 12, 2012, accessed March 16, 2015 .
- ↑ B. Sens, M. Stern (Ed.): Report volume: Quality assurance for cystic fibrosis .2012 ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. Hippocampus Verlag, ISBN 978-3-88579-906-1 , p. 39.
- ↑ a b M. Stopsack, J. Hammermann: Newborn screening for cystic fibrosis. In: Monthly Pediatrics. Volume 157, number 12, pp. 1222-1229 doi: 10.1007 / s00112-009-2042-6
- ↑ Lutz Nahrlich, Klaus-Peter Zimmer: Newborn Screening for Cystic Fibrosis: Let's Begin! In: Deutsches Ärzteblatt . tape 110 , no. 20 . Deutscher Ärzte-Verlag , May 17, 2013, p. 354–5 , doi : 10.3238 / arztebl.2013.0354 .
- ↑ Ingeborg Bördlein: Congenital metabolic diseases: Long-term study proves the benefit of newborn screening . In: Deutsches Ärzteblatt . tape 108 , no. 36 . Deutscher Ärzte-Verlag , September 9, 2011, p. A-1855 / B-1581 / C-1570 .
- ↑ Erik Harms, Bernhard Olgemöller: Newborn screening for metabolic diseases and endocrinopathies . In: Deutsches Ärzteblatt . tape 108 , no. 1-2 . Deutscher Ärzte-Verlag , January 10, 2011, p. 11–22 , doi : 10.3238 / arztebl.2011.0011 .
- ^ Udo Wendel, Martin Lindner, Markus Bettendorf: Newborn screening in Germany. Schattauer Verlag, 2009, ISBN 3-7945-2686-4 , p. 78 restricted preview in the Google book search
- ↑ Deutsches Ärzteblatt 33/34 of August 21, 2017
- ↑ DS Hardin: Growth problems and growth hormone treatment in children with cystic fibrosis. In: Journal of pediatric endocrinology & metabolism. Volume 15 Suppl 2, May 2002, pp. 731-735, PMID 12092687 (review).
- ↑ a b c d Tom O. Hirche, Thomas OF Wagner: Update Mukoviszidose. Georg Thieme Verlag, 2013, ISBN 978-3-13-176981-7 , pp. 11-21 ( limited preview in the Google book search)
- ↑ Marianne Abele-Horn (2009), pp. 193 and 195.
- ↑ DGPI manual. Georg Thieme Verlag, 2013, ISBN 978-3-13-166546-1 , p. 460 ( limited preview in the Google book search).
- ↑ Peter Hien: Practical Pneumology. Chapter 56: Cystic Fibrosis. 2nd edition, Springer-Verlag, 2011, ISBN 3-642-10209-3 , p. 185 ( limited preview in the Google book search)
- ↑ Christine Vetter: DNase for people with cystic fibrosis: lung function is significantly improved . In: Deutsches Ärzteblatt . tape 91 , no. 47 . Deutscher Ärzte-Verlag , November 25, 1994, p. A-3302 .
- ↑ DS Hardin, C. Ahn et al. a .: Growth hormone improves bone mineral content in children with cystic fibrosis. In: Journal of pediatric endocrinology & metabolism. Volume 18, Number 6, June 2005, pp. 589-595, PMID 16042327 .
- ^ V. Teichgräber, M. Ulrich u. a .: Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. In: Nature medicine. Volume 14, Number 4, April 2008, pp. 382-391, doi: 10.1038 / nm1748 , PMID 18376404 .
- ↑ New treatment for cystic fibrosis. (No longer available online.) In: science.orf.at. March 30, 2008, formerly in the original ; accessed on March 6, 2015 . ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ↑ J. Kornhuber, M. Muehlbacher u. a .: Identification of novel functional inhibitors of acid sphingomyelinase. In: PloS one. Volume 6, number 8, 2011, p. E23852, doi: 10.1371 / journal.pone.0023852 , PMID 21909365 , PMC 3166082 (free full text).
- ↑ TG Liou, FR Adler a. a .: Lung transplantation and survival in children with cystic fibrosis. In: The New England Journal of Medicine . Volume 357, number 21, November 2007, pp. 2143-2152, doi: 10.1056 / NEJMoa066359 , PMID 18032764 , PMC 2522236 (free full text).
- ↑ Peter Hien: Practical Pneumology. Chapter 56: Cystic Fibrosis. 2nd edition, Springer-Verlag, 2011, ISBN 3-642-10209-3 , pp. 479-484 ( limited preview in the Google book search)
- ↑ a b E. M. Welch, ER Barton a. a .: PTC124 targets genetic disorders caused by nonsense mutations. In: Nature. Volume 447, number 7140, May 2007, pp. 87-91, doi: 10.1038 / nature05756 , PMID 17450125 .
- ↑ GL McPhail, JP Clancy: Ivacaftor: the first therapy acting on the primary cause of cystic fibrosis. In: Drugs of today. Volume 49, number 4, April 2013, pp. 253-260, doi: 10.1358 / dot.2013.49.4.1940984 , PMID 23616952 .
- ↑ FDA approves Kalydeco to treat rare form of cystic fibrosis. In: fda.gov. January 31, 2012, accessed March 2, 2015 .
- ↑ Kalydeco - ivacaftor. In: ema.europa.eu. August 6, 2012, accessed March 2, 2015 .
- ↑ Summary of the EPAR for the public: Kalydeco - Ivacaftor. European Medicines Agency, July 2014 (PDF file; 61 kB)
- ↑ K. Kotha, JP Clancy: Ivacaftor treatment of cystic fibrosis patients with the G551D mutation: a review of the evidence. In: Therapeutic advances in respiratory disease. Volume 7, Number 5, October 2013, pp. 288-296, doi: 10.1177 / 1753465813502115 , PMID 24004658 (review).
- ↑ I. Sermet-Gaudelus: ivacaftor treatment in patients with cystic fibrosis and the G551D CFTR mutation. In: European respiratory review. Volume 22, number 127, March 2013, pp. 66-71, doi: 10.1183 / 09059180.00008512 , PMID 23457167 (review).
- ↑ a b c rme / aerzteblatt.de: Cystic fibrosis: Ivacaftor approved in Europe. In: aerzteblatt.de . July 27, 2012, accessed March 6, 2015 .
- ↑ a b muko.info: Ivacaftor (Kalydeco®; VX-770) approved in Germany. (No longer available online.) In: muko.info. November 29, 2012, archived from the original on April 2, 2015 ; accessed on March 6, 2015 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ A b Andrew Pollack: FDA Approves New Cystic Fibrosis Drug. In: nytimes.com. January 31, 2012, accessed March 6, 2015 .
- ↑ a b New Medicines 2012/2013 - a critical evaluation a critical evaluation. Lecture at a training event organized by the German Medicines Commission of the German Medical Association together with the Medical Association of Saxony-Anhalt and the KV Saxony-Anhalt April 27, 2013 in Halle (Saale); (PDF file)
- ↑ N. Derichs: Targeting a genetic defect: cystic fibrosis transmembrane conductance regulator modulators in cystic fibrosis. In: European respiratory review. Volume 22, number 127, March 2013, pp. 58–65, doi: 10.1183 / 09059180.00008412 , PMID 23457166 (review article, freely accessible in full).
- ↑ N. Lenherra, L. Krueger et al. a .: Ivacaftor - the first drug for the causal therapy of cystic fibrosis. In: Switzerland Med Forum. Volume 14, number 43, 2014, pp. 799-801.
- ↑ US Food and Drug Administration Approves KALYDECO ™ (ivacaftor) for Use in Eight Additional Mutations that Cause Cystic Fibrosis. In: investors.vrtx.com. February 21, 2014, accessed March 29, 2015 .
- ↑ Rüdiger Meyer: cystic fibrosis. A triple therapy is highly effective and superior to the two-way combination. In: Deutsche Ärzteblatt. Volume 116, Issue 49, December 6, 2019, SB 1892.
- ↑ JC Davies, EW Alton: Design of gene therapy trials in CF patients. In: Methods in molecular biology (Clifton, NJ). Volume 741, 2011, pp. 55-68, doi: 10.1007 / 978-1-61779-117-8_5 , PMID 21594778 (review).
- ↑ a b M. Wilschanski: Novel therapeutic approaches for cystic fibrosis. In: Discovery medicine. Volume 15, Number 81, February 2013, pp. 127-133, PMID 23449115 (review).
- ↑ I. Sermet-Gaudelus, KD Boeck u. a .: Ataluren (PTC124) induces cystic fibrosis transmembrane conductance regulator protein expression and activity in children with nonsense mutation cystic fibrosis. In: American journal of respiratory and critical care medicine. Volume 182, Number 10, November 2010, pp. 1262-1272, doi: 10.1164 / rccm.201001-0137OC , PMID 20622033 .
- ↑ Clinical study (phase III): Study of Ataluren in Nonsense Mutation Cystic Fibrosis (ACT CF) at Clinicaltrials.gov of the NIH
- ↑ MP Boyle, SC Bell et al. a .: A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomized controlled trial. In: The Lancet. Respiratory medicine. Volume 2, Number 7, July 2014, pp. 527-538, doi: 10.1016 / S2213-2600 (14) 70132-8 , PMID 24973281 .
- ↑ Clinical Study (Phase III): A Phase 3 Rollover Study of Lumacaftor in Combination With Ivacaftor in Subjects 12 Years and Older With Cystic Fibrosis at Clinicaltrials.gov of the NIH
- ↑ T. Iannitti, B. Palmieri: Clinical and experimental applications of sodium phenylbutyrate. In: Drugs in R&D. Volume 11, number 3, September 2011, pp. 227-249, doi: 10.2165 / 11591280-000000000-00000 , PMID 21902286 , PMC 3586072 (free full text) (review).
- ^ PL Zeitlin, M. Diener-West a. a .: Evidence of CFTR function in cystic fibrosis after systemic administration of 4-phenylbutyrate. In: Molecular therapy. Volume 6, number 1, July 2002, pp. 119-126, doi: 10.1006 / mthe.2002.0639 , PMID 12095312 .
- ↑ Ammonaps - Appendix I: Summary of Product Characteristics. Pp. 4-5.
- ↑ Clinical study (phase I): Phenylbutyrate / Genistein Duotherapy in Delta F508-Heterozygotes (for Cystic Fibrosis) at Clinicaltrials.gov of the NIH
- ↑ Clinical study (phase I + II): 'at Clinicaltrials.gov the NIH
- ↑ a b Public summary of opinion on orphan designation: 4,6,4'-Trimethylangelicin for the treatment of cystic fibrosis. Committee for Orphan Medicinal Products, July 8, 2013
- ↑ M. Favia, MT Mancini et al. a .: Trimethylangelicin promotes the functional rescue of mutant F508del CFTR protein in cystic fibrosis airway cells. In: American journal of physiology. Lung cellular and molecular physiology. Volume 307, number 1, July 2014, pp. L48-L61, doi: 10.1152 / ajplung.00305.2013 , PMID 24816489 .
- ↑ JF Collawn, L. Fu et al. a .: Rescuing ΔF508 CFTR with trimethylangelicin, a dual-acting corrector and potentiator. In: American journal of physiology. Volume 307, number 6, September 2014, pp. L431 – L434, doi: 10.1152 / ajplung.00177.2014 , PMID 25063802 , PMC 4166784 (free full text) (review).
- ↑ a b c T. S. Cohen, A. Prince: Cystic fibrosis: a mucosal immunodeficiency syndrome. In: Nature medicine. Volume 18, number 4, April 2012, pp. 509-519, doi: 10.1038 / nm.2715 , PMID 22481418 , PMC 3577071 (free full text) (review).
- ↑ LL Clarke, BR Grubb et al. a .: Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. In: Science. Volume 257, Number 5073, August 1992, pp. 1125-1128, PMID 1380724 .
- ↑ JN Snouwaert, KK Brigman u. a .: An animal model for cystic fibrosis made by gene targeting. In: Science. Volume 257, Number 5073, August 1992, pp. 1083-1088, PMID 1380723 .
- ↑ R. Rozmahel, M. Wilschanski u. a .: Modulation of disease severity in cystic fibrosis transmembrane conductance regulator deficient mice by a secondary genetic factor. In: Nature genetics. Volume 12, Number 3, March 1996, pp. 280-287, doi: 10.1038 / ng0396-280 , PMID 8589719 .
- ↑ CS Rogers, Y. Hao, et al. a .: Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. In: The Journal of clinical investigation. Volume 118, number 4, April 2008, pp. 1571-1577, doi: 10.1172 / JCI34773 , PMID 18324337 , PMC 2265103 (free full text).
- ↑ LS Ostedgaard, DK Meyerholz u. a .: The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. In: Science Translational Medicine . Volume 3, number 74, March 2011, p. 74ra24, doi: 10.1126 / scitranslmed.3001868 , PMID 21411740 , PMC 3119077 (free full text).
- ↑ DA Stoltz, DK Meyerholz u. a .: Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. In: Science Translational Medicine . Volume 2, number 29, April 2010, p. 29ra31, doi: 10.1126 / scitranslmed.3000928 , PMID 20427821 , PMC 2889616 (free full text).
- ↑ Y. Song, W. Namkung, et al. a .: Airway surface liquid depth measured in ex vivo fragments of pig and human trachea: dependence on Na + and Cl- channel function. In: American journal of physiology. Lung cellular and molecular physiology. Volume 297, number 6, December 2009, pp. L1131 – L1140, doi: 10.1152 / ajplung.00085.2009 , PMID 19820035 , PMC 2793186 (free full text).
- ↑ X. Sun, H. Sui et al. a .: Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. In: The Journal of clinical investigation. Volume 120, number 9, September 2010, pp. 3149-3160, doi: 10.1172 / JCI43052 , PMID 20739752 , PMC 2929732 (free full text).
- ^ NW Keizer, JF Engelhardt: New animal models of cystic fibrosis: what are they teaching us? In: Current opinion in pulmonary medicine. Volume 17, number 6, November 2011, pp. 478-483, doi: 10.1097 / MCP.0b013e32834b14c9 , PMID 21857224 , PMC 3596000 (free full text) (review).
- ^ PB Davis: Cystic fibrosis since 1938. In: American journal of respiratory and critical care medicine. Volume 173, Number 5, March 2006, pp. 475-482, doi: 10.1164 / rccm.200505-840OE , PMID 16126935 (review).
- ↑ JA Dodge, PA Lewis et al. a .: Cystic fibrosis mortality and survival in the UK: 1947-2003. In: The European respiratory journal. Volume 29, Number 3, March 2007, pp. 522-526, doi: 10.1183 / 09031936.00099506 , PMID 17182652 .
- ↑ LK Tuchman, LA Schwartz a. a .: Cystic fibrosis and transition to adult medical care. In: Pediatrics. Volume 125, number 3, March 2010, pp. 566-573, doi: 10.1542 / peds.2009-2791 , PMID 20176665 (review).
- ↑ Irmgard Eichler: Cystic fibrosis (cystic fibrosis). In: Christian P. Speer, Manfred Gahr (Ed.): Pediatrics. Springer-Verlag, 2013, ISBN 3-662-09178-X , pp. 558-565, limited preview in the Google book search
- ^ JL Kreindler, VA Miller: Cystic fibrosis: addressing the transition from pediatric to adult-oriented health care. In: Patient preference and adherence. Volume 7, 2013, pp. 1221-1226, doi: 10.2147 / PPA.S37710 , PMID 24376344 , PMC 3864992 (free full text) (review).
- ↑ BS Quon, WD Bentham et al. a .: Prevalence of Symptoms of Depression and Anxiety in Adults With Cystic Fibrosis Based on the PHQ-9 and GAD-7 Screening Questionnaires. In: Psychosomatics. [Electronic publication before printing] June 2014, doi: 10.1016 / j.psym.2014.05.017 , PMID 25556569 .
- ↑ N. Sharer, M. Schwarz et al. a .: Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. In: The New England Journal of Medicine . Volume 339, Number 10, September 1998, pp. 645-652, doi: 10.1056 / NEJM199809033391001 , PMID 9725921 .
- ↑ JA Cohn, KJ Friedman et al. a .: Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. In: The New England Journal of Medicine . Volume 339, Number 10, September 1998, pp. 653-658, doi: 10.1056 / NEJM199809033391002 , PMID 9725922 .
- ^ Tilman Horn: Pancreatitis in patients with hyperparathyroidism: Association with mutations in the SPINK1 and CFTR genes. Dissertation, Ruhr University Bochum, Faculty of Medicine, 2009, p. 47.
- ^ JO Warner, AP Norman, JF Soothill: Cystic fibrosis heterozygosity in the pathogenesis of allergy. In: The Lancet. Volume 1, Number 7967, May 1976, pp. 990-991, PMID 57442 .
- ↑ M. Dahl, A. Tybjaerg-Hansen u. a .: DeltaF508 heterozygosity in cystic fibrosis and susceptibility to asthma. In: The Lancet. Volume 351, Number 9120, June 1998, pp. 1911-1913, PMID 9654257 .
- ↑ C. Lázaro, R. de Cid et al. a .: Missense mutations in the cystic fibrosis gene in adult patients with asthma. In: Human mutation. Volume 14, Number 6, 1999, pp. 510-519, doi : 10.1002 / (SICI) 1098-1004 (199912) 14: 6 <510 :: AID-HUMU10> 3.0.CO; 2-O , PMID 10571949 .
- ↑ SA Schroeder, DM Gaughan, M. Swift: Protection against bronchial asthma by CFTR delta F508 mutation: a heterozygous advantage in cystic fibrosis. In: Nature medicine. Volume 1, Number 7, July 1995, pp. 703-705, PMID 7585155 .
- ↑ N. Maurya, S. Awasthi, P. Dixit: Association of CFTR gene mutation with bronchial asthma. In: The Indian journal of medical research. Volume 135, Number 4, April 2012, pp. 469-478, PMID 22664493 , PMC 3385229 (free full text) (review).
- ↑ a b T. A. VanWort, JA Lee u. a .: Female cystic fibrosis mutation carriers and assisted reproductive technology: does carrier status affect reproductive outcomes? In: Fertility and sterility. Volume 102, number 5, November 2014, pp. 1324-1330, doi: 10.1016 / j.fertnstert.2014.07.1234 , PMID 25217870 .
- ^ A b I. Gallego Romero, C. Ober: CFTR mutations and reproductive outcomes in a population isolate. In: Human genetics. Volume 122, Number 6, January 2008, pp. 583-588, doi: 10.1007 / s00439-007-0432-1 , PMID 17901983 .
- ↑ M. Dahl, A. Tybjaerg-Hansen u. a .: Cystic fibrosis Delta F508 heterozygous, smoking, and reproduction: studies of 9141 individuals from a general population sample. In: Genomics. Volume 50, number 1, May 1998, pp. 89-96, doi: 10.1006 / geno.1998.5272 , PMID 9628826 .
- ^ SV Raju, JH Tate et al. a .: Impact of heterozygous CFTR mutations in COPD patients with chronic bronchitis. In: Respiratory research. Volume 15, 2014, p. 18, doi: 10.1186 / 1465-9921-15-18 , PMID 24517344 , PMC 3925354 (free full text).
- ↑ CR Lake, PB Davis et al. a .: Electrolytes and norepinephrine levels in blood of patients with cystic fibrosis. In: Clinica Chimica Acta . Volume 92, Number 2, March 1979, pp. 141-146, PMID 487568 .
- ↑ a b c M. Super, A. Irtiza-Ali u. a .: Blood pressure and the cystic fibrosis gene: evidence for lower pressure rises with age in female carriers. In: Hypertension. Volume 44, Number 6, December 2004, pp. 878-883, doi: 10.1161 / 01.HYP.0000145901.81989.46 , PMID 15477385 .
- ↑ J. Lieberman, S. Rodbard: Low blood pressure in young adults with cystic fibrosis: an effect of chronic salt loss in sweat? In: Annals of internal medicine. Volume 82, Number 6, June 1975, pp. 806-808, PMID 1138590 .
- ↑ JJ Guo, DA Stoltz a. a .: Genotype-specific alterations in vascular smooth muscle cell function in cystic fibrosis piglets. In: Journal of cystic fibrosis. Volume 13, number 3, May 2014, pp. 251-259, doi: 10.1016 / j.jcf.2013.10.009 , PMID 24183914 , PMC 3972271 (free full text).
- ↑ VA Peotta, P. Bhandary u. a .: Reduced blood pressure of CFTR-F508del carriers correlates with diminished arterial reactivity rather than circulating blood volume in mice. In: PloS one. Volume 9, number 5, 2014, p. E96756, doi: 10.1371 / journal.pone.0096756 , PMID 24801204 , PMC 4011854 (free full text).
- ↑ N. Morral, V. Nunes et al. a .: Microsatellite haplotypes for cystic fibrosis: mutation frameworks and evolutionary tracers. In: Human molecular genetics. Volume 2, Number 7, July 1993, pp. 1015-1022, PMID 7689896 .
- ↑ S. Rendine, F. Calafell et al. a .: Genetic history of cystic fibrosis mutations in Italy. I. Regional distribution. In: Annals of Human Genetics. Volume 61, 1997, pp. 411-424, doi: 10.1046 / j.1469-1809.1997.6150411.x , PMID 9459003 .
- ↑ C. Wiuf: Do delta F508 heterozygotes have a selective advantage? In: Genetical research. Volume 78, Number 1, August 2001, pp. 41-47, PMID 11556136 .
- ↑ Heike Bickeböller, Christine Fischer: Introduction to Genetic Epidemiology. Springer-Verlag, 2007, ISBN 3-540-33568-4 , p. 73 limited preview in the Google book search
- ^ M. Claustres: Molecular pathology of the CFTR locus in male infertility. In: Reproductive biomedicine online. Volume 10, Number 1, January 2005, pp. 14-41, PMID 15705292 (review).
- ^ SE Gabriel, KN Brigman u. a .: Cystic fibrosis heterozygous resistance to cholera toxin in the cystic fibrosis mouse model. In: Science. Volume 266, Number 5182, October 1994, pp. 107-109, PMID 7524148 .
- ^ GB Pier, M. Grout u. a .: Salmonella typhi uses CFTR to enter intestinal epithelial cells. In: Nature. Volume 393, Number 6680, May 1998, pp. 79-82, doi: 10.1038 / 30006 , PMID 9590693 .
- ↑ JB Lyczak, GB Pier: Salmonella enterica serovar typhi modulates cell surface expression of its receptor, the cystic fibrosis transmembrane conductance regulator, on the intestinal epithelium. In: Infection and Immunity. Volume 70, Number 11, November 2002, pp. 6416-6423, PMID 12379722 , PMC 130400 (free full text).
- ↑ E. van de Vosse, S. Ali et al. a .: Susceptibility to typhoid fever is associated with a polymorphism in the cystic fibrosis transmembrane conductance regulator (CFTR). In: Human genetics. Volume 118, Number 1, October 2005, pp. 138-140, doi: 10.1007 / s00439-005-0005-0 , PMID 16078047 .
- ↑ E. van de Vosse, AW de Visser and a .: Distribution of CFTR variations in an Indonesian enteric fever cohort. In: Clinical infectious diseases. Volume 50, Number 9, May 2010, pp. 1231-1237, doi: 10.1086 / 651598 , PMID 20233062 .
- ↑ a b c d e E. M. Poolman, AP Galvani: Evaluating candidate agents of selective pressure for cystic fibrosis. In: Journal of the Royal Society. Volume 4, number 12, February 2007, pp. 91-98, doi: 10.1098 / rsif.2006.0154 , PMID 17015291 , PMC 2358959 (free full text).
- ↑ CM Anderson, J. Allan, PG Johansen: Comments on the possible existence and nature of a heterozygous advantage in cystic fibrosis. In: Bibliotheca paediatrica. Volume 86, 1967, pp. 381-387, PMID 6054652 .
- ^ MA Crawfurd: A genetic study, including evidence for heterosis, of cystic fibrosis of the pancreas. In 168th Meeting of the Genetical Society of Great Britain, 1972, p. 126.
- ↑ JK Tobacman: Does deficiency of arylsulfatase B have a role in cystic fibrosis? In: Chest. Volume 123, Number 6, June 2003, pp. 2130-2139, PMID 12796199 (review).
- ^ AP Galvani, M. Slatkin: Evaluating plague and smallpox as historical selective pressures for the CCR5-Delta 32 HIV-resistance allele. In: Proceedings of the National Academy of Sciences . Volume 100, number 25, December 2003, pp. 15276-15279, doi: 10.1073 / pnas.2435085100 , PMID 14645720 , PMC 299980 (free full text).
- ↑ AL Hughes, R. Friedman, M. Murray: Genomewide pattern of synonymous nucleotide substitution in two complete genomes of Mycobacterium tuberculosis. In: Emerging infectious diseases. Volume 8, number 11, November 2002, pp. 1342-1346, doi: 10.3201 / eid0811.020064 , PMID 12453367 , PMC 2738538 (free full text).
- ^ G. Modiano, BM Ciminelli, PF Pignatti: Cystic fibrosis and lactase persistence: a possible correlation. In: European journal of human genetics. Volume 15, Number 3, March 2007, pp. 255-259, doi: 10.1038 / sj.ejhg.5201749 , PMID 17180122 (review).
- ↑ D. Saleheen, PM Frossard: The cradle of the deltaF508 mutation. In: Journal of Ayub Medical College, Abbottabad. Volume 20, Number 4, 2008, pp. 157-160, PMID 19999232 (review).
- ↑ a b c Gerhard Dockter, Hermann Lindemann: introduction and basics. In: Hermann Lindemann, Burckhardt Tümmler, Gerhard Dockter (ed.): Mukoviszidose - Cystic Fibrosis. 4th edition, Georg Thieme, 2004, ISBN 3-13-138604-5 , pp. 1–2, restricted preview in the Google book search
- ↑ a b Ulrich Grossekettler: Increased serum concentrations of IL-18 and IL-12 p40 in cystic fibrosis Dissertation, Johann Wolfgang Goethe University Frankfurt am Main, 2005, p. 5 restricted preview in the Google book search
- ^ R. Busch: On the history of cystic fibrosis. In: Acta Universitatis Carolinae. Medica. Volume 36, Numbers 1-4, 1990, pp. 13-15, PMID 2130674 (review).
- ^ A b James M. Littlewood: History of cystic fibrosis. In: Margaret Hodson, Andrew Bush, Duncan Geddes: Cystic Fibrosis. 3rd edition, CRC Press, 2012, ISBN 978-1-4441-1369-3 , pp. 3-19 ( limited preview in the Google book search).
- ^ Ernst Ludwig Rochholz : Alemannic children's song and children's game from Switzerland JJ Weber, Leipzig, 1857 limited preview in the Google book search
- ↑ M. Griese, D. Reinhardt: Differential diagnosis of pathological sweat test results. In: Dietrich Reinhardt, Manfred Götz, Richard Kraemer, Martin H. Schöni (Eds.): Cystic Fibrosis. Springer-Verlag, 2013, ISBN 978-3-642-56796-4 , p. 212 ( limited preview in Google book search).
- ↑ C. Falkman: Cystic fibrosis - a psychological study of 52 children and their families. In: Acta paediatrica Scandinavica. Supplement. Number 269, 1977, pp. 1-93, PMID 274900 .
- ↑ JX Pfyffer: Quoting from the dictionary of the Swiss German language Volume 7, 1848, p. 899. See: James L. Littlewood: History of cystic fibrosis. In: Margaret Hodson, Andrew Bush, Duncan Geddes: Cystic Fibrosis. 3rd edition, CRC Press, 2012, ISBN 978-1-4441-1369-3 , 486 pp. ( Limited preview in Google book search)
- ^ G. Fanconi, E. Uehlinger, C. Knauer: The celiac syndrome in congenital cystic pancreatic fibrosis and bronchiectasis. In: Wien Med Wochenschr. Volume 86, 1936, pp. 753-756.
- ↑ U. Stephan, M. Götz, K. Stephan, S. Bender: Cystic Fibrosis. In: P. Frick, G.-A. von Harnack, GA Martini, A. Prader, HP Wolff (Eds.): Advances in Internal Medicine and Pediatrics. Volume 44, Springer Berlin Heidelberg, 1980, ISBN 978-3-642-67559-1 , pp. 73-174 doi: 10.1007 / 978-3-642-67557-7_3
- ↑ a b K. Landsteiner: Intestinal obstruction due to thickened meconium: pancreatitis. In: Centralblatt for general pathology and pathological anatomy. Volume 16, 1905, p. 903.
- ↑ a b Ralf Bröer: A pediatrician who was strict, curious and humane. In: aerztezeitung.de. October 11, 2004, accessed March 12, 2015 .
- ^ Friedrich Katscher: Hope for people with cystic fibrosis. In: wienerzeitung.at. November 26, 1999, accessed March 12, 2015 .
- ↑ DH Andersen: Cystic fibrosis of the pancreas and its relation to celiac disease. In: American Journal of Diseases of Children. Volume 56, 1938, pp. 344-399.
- ↑ S. Zabransky, S. Isabel Zink: IRT determination in whole blood samples dried on filter paper as a search test for cystic fibrosis In: Siegfried Zabransky (Ed.): Screening for congenital endocrine and metabolic disorders. 2001, pp. 269-296 doi: 10.1007 / 978-3-7091-6252-1_33 , ISBN 978-3-7091-7260-5
- ^ S. Farber: Pancreatic function and disease in early life. V. Pathology changes associated with pancreatic insufficiency in early life. In: Arch Path. Volume 37, 1944, p. 238.
- ^ CU Lowe, CD May, SC Reed: Fibrosis of the pancreas in infants and children; a statistical study of clinical and hereditary features. In: American journal of diseases of children. Volume 78, Number 3, September 1949, pp. 349-374, doi: 10.1001 / archpedi.1949.02030050362008 , PMID 18138931 .
- ^ RC Darling, PA di Sant'Agnese u. a .: Electrolyte abnormalities of the sweat in fibrocystic disease of the pancreas. In: The American journal of the medical sciences. Volume 225, Number 1, January 1953, pp. 67-70, PMID 13007698 .
- ^ LE Gibson, RE Cooke: A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. In: Pediatrics. Volume 23, Number 3, March 1959, pp. 545-549, PMID 13633369 .
- ^ PM Quinton: Chloride impermeability in cystic fibrosis. In: Nature. Volume 301, Number 5899, February 1983, pp. 421-422, PMID 6823316 .
- ^ RG Knowlton, O. Cohen-Haguenauer u. a .: A polymorphic DNA marker linked to cystic fibrosis is located on chromosome 7. In: Nature. Volume 318, Number 6044, 1985, pp. 380-382, PMID 2999611 .
- ↑ JM Rommens, MC Iannuzzi u. a .: Identification of the cystic fibrosis gene: chromosome walking and jumping. In: Science. Volume 245, Number 4922, September 1989, pp. 1059-1065, PMID 2772657 .
- ^ WK Lemna, GL Feldman a. a .: Mutation analysis for heterozygous detection and the prenatal diagnosis of cystic fibrosis. In: The New England Journal of Medicine . Volume 322, Number 5, February 1990, pp. 291-296, doi: 10.1056 / NEJM199002013220503 , PMID 2296270 . (Full text freely accessible)
- ^ Rare Diseases
- ↑ Cystic fibrosis with gastritris and megaloblastic anemia. In: Orphanet (Rare Disease Database).