Xanthotoxin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
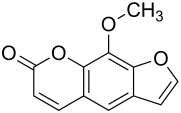
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Methoxsalen | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 12 H 8 O 4 | |||||||||||||||||||||
| Brief description |
colorless needles or prisms, tastes bitter with a tingling aftertaste |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 216.18 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
148 ° C |
|||||||||||||||||||||
| solubility |
soluble in acetone , boiling alcohol; sparingly soluble in hot water , ether |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Xanthotoxin is a natural substance found in the essential oils of various plants . Xanthotoxin is a substance from the group of psoralens , which in turn are counted among the coumarins .
Occurrence

Xanthotoxin occurs in the African tree Fagara xanthoxyloides and in various umbelliferae such as angelica , bergamot , parsnip and giant hogweed .
Clinical information
Application areas (indications)
The drug is used for some skin diseases (e.g. psoriasis ). At the same time, long-wave UV light can also be used. In such a case, one speaks of PUVA therapy.
hazards
Xanthotoxin is poisonous for cold blooded animals . Like the chemically related bergapten , xanthotoxin, being a linear furanocoumarin, has photosensitizing properties, i.e. it sensitizes the skin to sunlight and UV radiation. The substance causes severe inflammation and sunburn when exposed to light . Xanthotoxin also damages the DNA of the skin cells, so that long-term damage such as cancer can result.
Trade names
Meladinine (D, CH), Oxsoralen (A), Uvadex (D, A, CH)
literature
- Ernst Mutschler: drug effects. 7th edition 1996, WVG Stuttgart, ISBN 3-8047-1377-7
Individual evidence
- ↑ a b c d Entry on xanthotoxin. In: Römpp Online . Georg Thieme Verlag, accessed on March 15, 2011.
- ↑ a b Xanthotoxin data sheet from Sigma-Aldrich , accessed on May 13, 2017 ( PDF ).
