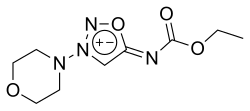
Molsidomine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Mesomeric boundary structures | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Molsidomine | |||||||||||||||||||||
| other names |
N - (ethoxycarbonyl) -3- (4-morpholinyl) sydnonimine |
|||||||||||||||||||||
| Molecular formula | C 9 H 14 N 4 O 4 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
Vascular dilatation by splitting off nitric oxide |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 242.2 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
140-141 ° C |
|||||||||||||||||||||
| pK s value |
3.0 |
|||||||||||||||||||||
| solubility |
Slightly soluble in water, soluble in dilute hydrochloric acid |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Molsidomine belongs to the group of non-enzymatic nitric oxide donors . This drug releases nitric oxide (NO) and thus leads to vascular dilation with a reduction in the wall tension of the coronary arteries. It is therefore used for the treatment of angina pectoris in coronary heart disease and for the treatment of acutely decompensated heart failure .
Molsidomine was first synthesized in 1970 by K. Masuda at Takeda . In the same year, K. Kikuchi observed the antihypertensive and antianginal effects.
Pharmacokinetics
Molsidomine is an inactive precursor ( prodrug ). It is not itself responsible for the vasodilating effect, but is metabolized in the liver to linsidomine (SIN-1) . This changes into the open-ring form without the action of enzymes and the actual active substance nitrogen monoxide is split off. Since no enzymes are required for the formation of free nitrogen monoxide, there is no weakening of the effect even with long-term administration ( nitrate tolerance ). Molsidomine inhibits platelet adhesion to a small extent . However, this effect is of no clinical relevance due to the small effect .
Molsidomine → Linsidomine (SIN-1) → SIN-1A → Nitric oxide
Pharmacodynamics
The release of nitric oxide leads to a dilatation of the coronary arteries, the large vena cava and the pulmonary vessels, thereby reducing the load on the heart. According to the Frank Starling mechanism , this leads to a decrease in the blood filling of the heart, the heart wall is no longer stretched as much and thus oxygen consumption is reduced. Because the more the heart wall is stretched, the more work it does. The widening of the coronary vessels and the decrease in filling lead to better blood flow to the heart wall and thus to an improvement in the oxygen supply to the heart muscles .
The directly active substance, linsidomine, is also available for direct intravenous therapy.
application
Molsidomin is therapeutically prescribed for the prophylaxis of angina pectoris attacks. Acute therapy is not possible with molsidomine, as the maximum effect is only achieved after 30 to 60 minutes.
Side effects
The most important side effects are headache, dizziness and a drop in blood pressure due to the severe vasodilation.
In animal experiments with rats, higher doses of molsidomine resulted in an increased number of malignant nasal tumors.
Trade names
Corvaton (D, CH), Molsibeta (D), Molsidolat (A), Molsiket (D), Molsi-Puren (D), various generics (D)
Individual evidence
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition. 2006, ISBN 0-911910-00-X , pp. 1076-1077.
- ↑ a b c d e entry on molsidomine. In: Römpp Online . Georg Thieme Verlag, accessed on July 16, 2019.
- ↑ a b Molsidomine data sheet from Sigma-Aldrich , accessed on April 10, 2011 ( PDF ).
- ↑ German Medical Association (BÄK), National Association of Statutory Health Insurance Physicians (KBV), Working Group of Scientific Medical Societies (AWMF): National Care Guideline for Chronic Heart Failure - Long Version. 2017; Version 2.
- ^ Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2., revised. and exp. Ed. Wiss. Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 163 .
- ↑ Red List online, as of September 2009.
- ↑ Am Comp. d. Switzerland, as of September 2009.
- ↑ AGES-Pharmmed, as of September 2009.
literature
- B. Rosenkranz, BR Winkelmann, MJ Parnham: Clinical Pharmacokinetics of molsidomine. In: Clin Pharmacokinet . (30), 1996, pp. 372-384. PMID 8743336
- Product information from Cayman Chemical Company. (English; PDF file; 153 kB)
- Ulrich Förstermann et al. (Hrsg.): General and special pharmacology and toxicology. Urban & Fischer, 2006, ISBN 3-437-44490-5 .
- Technical information from Corvaton ®