Musk xylene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Musk xylene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 15 N 3 O 6 | |||||||||||||||
| Brief description |
yellowish crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 297.28 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
violent decomposition |
|||||||||||||||
| solubility |
soluble in ethanol |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Authorization procedure under REACH |
of particular concern : very persistent and very bioaccumulative ( vPvB ); subject to approval |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
5- tert -Butyl-2,4,6-trinitro- m -xylene (also muskxylene for short ) is a chemical compound from the group of aromatic nitro compounds .
history
Musk xylene - like musk ketone , musk ambrette, which was banned in 1995, and other related fragrances - was discovered by the chemist Albert Baur (1856–1933) and brought onto the market.
Extraction and presentation
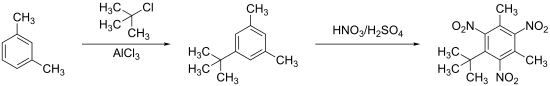
Musk xylene is obtained from m- xylene by Friedel-Crafts alkylation with tert-butyl chloride and aluminum chloride and subsequent nitration with fuming nitric acid or with a 70:30 mixture of nitric acid and sulfuric acid.
properties
Musk xylene is a yellowish solid. It decomposes violently when heated, producing nitrous gases . When dissolved in ethanol , the compound has a strong, pleasant smell of musk .
use
Musk xylene was used in large quantities as a fragrance, for example 86 tons of it were processed in Europe in 1998. Due to its very high persistence and bioaccumulativity ( vPvB ), musk xylene was identified as an SVHC substance under REACH and included in Annex XIV of the substances subject to authorization. Since August 2014, its use is no longer permitted in the EU.
Web links
Individual evidence
- ↑ a b c d e Entry on 5-tert-butyl-2,4,6-trinitro-m-xylene in the GESTIS substance database of the IFA , accessed on May 14, 2017(JavaScript required) .
- ↑ a b c Michael Woock, Michael W. Tausch: Synthesis of xylene musk (5-tert-butyltrinitro-m-xylene) ( Memento from January 6, 2013 in the Internet Archive )
- ↑ Entry on 5-tert-butyl-2,4,6-trinitro-m-xylene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet Musk xylene solution from Sigma-Aldrich , accessed on May 14, 2017 ( PDF ).
- ↑ a b Entry in the SVHC list of the European Chemicals Agency , accessed on July 14, 2014.
- ↑ a b Entry in the register of substances subject to authorization of the European Chemicals Agency , accessed on July 14, 2014.
- ↑ a b Wolfgang Legrum: Fragrances, between stench and fragrance: Occurrence, properties and use of fragrances and their mixtures . 2., revised. and exp. Springer Fachmedien Wiesbaden, Wiesbaden 2015, ISBN 978-3-658-07309-1 , p. 166–167 , doi : 10.1007 / 978-3-658-07310-7 ( limited preview in Google book search).