Iodine nitrogen
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
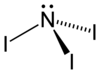
|
|||||||||||||
| General | |||||||||||||
| Surname | Iodine nitrogen | ||||||||||||
| other names |
Nitrogen triiodide |
||||||||||||
| Molecular formula | NI 3 | ||||||||||||
| Brief description |
black-brown solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 394.7 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| solubility |
decomposes in water |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Iodine nitrogen (NI 3 ), also known as nitrogen triiodide or chemically correct with iodine nitride , is an extremely labile chemical compound of iodine and nitrogen , which shows a strongly exothermic reaction ( explosion ) even with the slightest input of energy due to friction, impact or vibration .
Nitrogen triiodide belongs to the group of nitrogen halides .
synthesis
In pure form, iodine nitrogen was first synthesized in 1990 from boron nitride and iodine fluoride at −30 ° C in trichlorofluoromethane .
It is more common to display it by introducing iodine vapor or adding iodine crystals to a concentrated aqueous ammonia solution. The nitrogen is halogenated (only formally analogous to the hydrocarbons ). This leads to the formation of a polymeric nitrogen triiodide-ammonia adduct, usually referred to as iodine-nitrogen, NI 3 · NH 3 . This forms black-brown, rhombic crystals which decompose in water to form hydrogen iodide , iodine and ammonia.
The product is highly explosive and must be handled with the necessary care.
properties
Dry iodine nitrogen decomposes explosively with the slightest mechanical impact. Even a light touch with a bird's feather triggers the explosion. Spontaneous explosions also occur. The explosion always occurs with a very sharp bang. Great care must be taken with the dry solid. In contrast to the dried solid, it is safe to work with alcohol-moist iodine nitrogen, which makes this substance a popular prank for chemists in smaller quantities.
Ammonia adducts
Depending on the temperature and solvent, iodine-nitrogen forms differently colored polymeric adducts with ammonia corresponding to the hydrates :
- at temperatures from −30 to −15 ° C, a black-brown precipitate with the composition (NI 3 · NH 3 ) n is formed by adding iodine to a concentrated ammonia solution ;
- at −35 to −70 ° C a green, iridescent, poorly soluble polymer of the formula (NI 3 · 3 NH 3 ) n is formed in liquid ammonia ;
- At −75 to −95 ° C, a red precipitate of a polymer (NI 3 · 5 NH 3 ) n forms in a 2: 1 mixture of chloroform and ammonia .
The latter two adducts can be converted into one another by varying the temperature. All polymers consist of chains of NI 4 - tetrahedra which are additionally surrounded by ammonia molecules.
use
Because of its instability, iodine nitrogen has no practical use as an explosive . The synthesis of very small quantities is interesting for didactic illustration in the context of chemistry lessons, but is explicitly prohibited in Germany's schools. The dried compound is very delicate and immediately disintegrates when touched or heated with a spectacular bang accompanied by a purple cloud of iodine.
Web links
- Synthesis instructions (PDF file; 35 kB)
- Video with a detonation attempt
- Video
Individual evidence
- ↑ a b Entry on iodine nitrogen. In: Römpp Online . Georg Thieme Verlag, accessed on July 15, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ HP Latscha, HA Klein: Inorganische Chemie , 2002, p. 312ff, Springer, ISBN 3-540-42938-7
- ↑ J. Jander: Non-Aqueous Solvents for Preparation and Reactions of Nitrogen Halogen Compound (PDF; 465 kB), Pure Appl. Chem. , Vol. 49, pp. 67-73, Pergamon, 1977
- ↑ Guideline on safety in teaching , resolution of the KMK of September 9, 1994 as amended on June 14, 2019, p. 85, accessed on May 10, 2020.


