2- (2- (4-methyl-3-cyclohexen-1-yl) propyl) cyclopentanone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
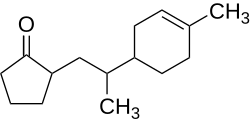
|
|||||||||||||||||||
| Figure without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2- [2- (4-methyl-3-cyclohexen-1-yl) propyl] cyclopentanone ( IUPAC ) | ||||||||||||||||||
| other names |
METHYLCYCLOHEXENYLPROPYL-CYCLOPENTANONE ( INCI ) |
||||||||||||||||||
| Molecular formula | C 15 H 24 O | ||||||||||||||||||
| Brief description |
viscous liquid with a fruity odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 220.35 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.96 g cm −3 (22 ° C) |
||||||||||||||||||
| Melting point |
−41.8 ° C |
||||||||||||||||||
| boiling point |
288 ° C |
||||||||||||||||||
| Vapor pressure |
16.13 hPa (20 ° C) |
||||||||||||||||||
| solubility |
practically insoluble in water (4.6 mg l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2- [2- (4-Methyl-3-cyclohexen-1-yl) propyl] cyclopentanone ( trade name of Givaudan : Nectaryl ) is an organic compound from the group of ketones and cycloalkenes . The compound is used as an odorant .
presentation
The compound is prepared by the radical addition of cyclopentanone to (+) - limonene under an oxygen atmosphere in acetic acid . As catalysts are manganese (II) acetate and cobalt (II) acetate used.
properties
The flash point of the compound is 162.5 ° C, the ignition temperature is 294 ° C. The specific angle of rotation is given as [α] D 20 = + 228-235 ° (1 M ; chloroform ).
The compound generally has a fruity, apricot-like odor. Of the four stereoisomers , (2 R , 2 ' S , 1' ' R ) -nectaryl and (2 R , 2' R , 1 '' R ) -nectaryl contribute to the odor of the compound, the odor threshold is 0.094 ng · L −1 or 0.112 ng · l −1 . In contrast to this, the other stereoisomers have an unspecific, fruity odor; the odor thresholds are significantly higher at 11.2 ng · l −1 and 14.9 ng · l −1 .
The tenacity on blotter (in German roughly: time spent on blotting paper ; the time during which the substance can be smelled with unchanged characteristics) is given as three weeks.
use
The substance is used as an odorous substance, for example in air care products, perfumes and polishes.
Individual evidence
- ↑ a b c d e f g h i Entry on 2- [2- (4-methyl-3-cyclohexen-1-yl) propyl] cyclopentanone in the GESTIS substance database of the IFA , accessed on April 3, 2019 (JavaScript required)
- ↑ a b Product Specification. (PDF) Nectaryl. Vigon, p. 8 , accessed on March 14, 2019 .
- ↑ a b c Elisabetta Brenna, Claudio Fuganti, Francesco G. Gatti, Luciana Malpezzi, Stefano Serra: Synthesis and olfactory evaluation of all stereoisomers of the fragrance Nectaryl . In: Tetrahedron: Asymmetry . tape 19 , no. 7 , April 2008, p. 800-807 , doi : 10.1016 / j.tetasy.2008.03.011 .
- ^ John C. Leffingwell: Chirality & Odor Perception. The Nectaryls. In: leffingwell.com. Retrieved April 3, 2019 .
- ↑ Wolfgang Legrum: Fragrances, between stench and fragrance . 2nd Edition. Springer, 2015, ISBN 978-3-658-07310-7 , chap. 3 , p. 51 .
- ↑ Wolfgang Legrum: Fragrances, between stench and fragrance . 2nd Edition. Springer, 2015, ISBN 978-3-658-07310-7 , chap. 3 , p. 52 ( limited preview in Google Book Search [accessed April 3, 2019]).
- ↑ InfoCard for 2- (2- (4-methyl-3-cyclohexen-1-yl) propyl) cyclopentanone from the European Chemicals Agency (ECHA), accessed on April 3, 2019.
