Neurobiological Schizophrenia Concepts
Under the heading of neurobiological schizophrenia concepts , facts and theories are summarized that deal with the models of schizophrenia as a disease , which are primarily created by natural scientists . Modern medicine researches the etiology and pathogenesis of schizophrenia at great expense and conducts studies in many areas. The ones presented here primarily concern genetics , neurochemistry , neuropharmacology , morphological findings and other organic factors.
genetics
Historical aspects
The tradition of modern genetic research in the field of schizophrenia goes back to the controversial German geneticist Ernst Rüdin . For many years Rüdin was head of the German Research Institute for Psychiatry (DFA for short), the predecessor organization of the Max Planck Institute for Psychiatry in Munich. The DFA was founded in 1917 on the initiative of Emil Kraepelin and incorporated into the Kaiser Wilhelm Society in 1924 . Rüdin was from 1918 head of the genealogical-demographic department of the DFA and from 1931 managing director of the entire DFA. Due to his influential work on the so-called law for the prevention of hereditary offspring, he is primarily responsible on the medical side for the forced sterilization of several hundred thousand people during the National Socialist era . The argumentative basis for this legislation and its criminal practice was, among other things, the empirical studies carried out by Rüdin and others on the hereditary nature of mental illnesses. Rüdin is considered an internationally recognized pioneer at the time.
Population Genetic Studies
The most basic statements about the population genetics of schizophrenia concern familial accumulation, twin studies, and adoption studies of the disease. Although 80% of schizophrenias occur sporadically, the risk of the disease is significantly higher in first-degree relatives (parents, children, siblings) of those with schizophrenia.
| Period | Number of studies |
relative | Incidence |
|---|---|---|---|
|
|
|
|
With a lifetime risk of around 1% for the average population, the risk of a schizophrenic sibling is around 10%, that of a dizygoti twin sibling around 14% and that of an identical twin sibling around 46%. The concordance rates for identical twins are high, but still well below one hundred percent, which suggests a genetic component of the disease, but also suggests that environmental factors contribute to the cause of schizophrenia.
| study | Matching MZ pairs |
Matching double room pairs |
|---|---|---|
|
|
|
Finally, the adoption studies on schizophrenia have shown that the risk of the disease does not depend on the parenting style of the birth or foster parents.
| Subjects | Schizophrenia cases among biological relatives |
Schizophrenia cases in the adoptive family |
|---|---|---|
|
|
|
These data show, in some cases, large differences from study to study, which are presumably largely due to the study design. However, all studies on the question of familial accumulation of schizophrenia show a trend towards a significantly increased risk depending on the degree of relationship. The mode of inheritance of schizophrenia is unclear. It is not certain whether there is a difference between the familial and the sporadic form of schizophrenia.
Human genetics of schizophrenia
Numerous studies have been carried out on the question of the molecular genetics of schizophrenia. The genetic studies are essentially based on two approaches: coupling studies and association studies . The principle of coupling is based on the fact that a “ disease gene ” is coupled with a “ marker gene ”. This simply means that the corresponding genes are closely spaced on a chromosome. Coupling analyzes can be used sensibly in diseases with Mendelian inheritance . In association studies one looks for any sequence variants that are inherited together with a characteristic. This type of genetic study is preferred for examinations to determine whether a suspected candidate gene is associated with a disease. A genetic connection with a disease can also exist in a non-coding gene segment. Association studies are less informative than coupling studies. To overview:
An overview of the most recent work on the genetics of schizophrenia shows that 6 genes or gene regions are considered promising candidates for a "schizophrenia gene":
- The dysbindin gene (DTNBP1) is located on chromosome 6p22. 3. It is found mainly in the area of the cerebellum and the hippocampus in postsynaptic structures. It may alter glutamate's presynaptic function. There is evidence of reduced expression of the dysbindin gene in schizophrenics.
- The gene for neuregulin 1 (NRG-1) is on chromosome 8p21. The NRG-1 gene is very large (over 1 Mb) and codes for more than 15 proteins. It contains 6 regulatory genes alone. In schizophrenics, a mutation in the regulatory gene IV of NRG-1 may be responsible for an altered expression of this NRG-1 subtype. Some recent studies suggest that changes in NRG-1 and its ErbB4 receptor increase the risk of developing schizophrenia.
- The gene for DISC1 was identified in a family with schizophrenia in which a balanced translocation t (1, 11) (q42; q14. 3) was found. In this area, two genes are destroyed by the translocation: DISC1 and DISC2. DISC2 does not contain any coding sections. However, it may regulate the expression of DISC1 through the formation of a specific antisense RNA. DISC1 is possibly responsible for processes of neuronal migration as it binds to parts of the neuronal cytoskeleton.
- The gene for DAOA ( D -amino acid oxidase activator, formerly G72) is on chromosome 13q22. 34. DAOA is only expressed in primates around the caudate nucleus and amygdala. As the name suggests, it activates DAO ( D -amino acid oxidase), which oxidizes D -serine, which in turn is an activator of the NMDA glutamate receptor. Various studies have found that some DAOA polymorphisms are associated with an increased risk of schizophrenia. DAOA is considered a “weaker” candidate, i.e. NRG-1 and DISC 1.
- The gene for COMT (catechol- O- methyltransferase) is located on chromosome 22q11 . COMT has a key role in the metabolism of catecholamines. In the synaptic cleft it breaks down dopamine into homovanillic acid and methoxythyramine. There are two forms of COMT: a soluble form (S-COMT) and a membrane-bound form (MB-COMT). If S-COMT has a valine instead of a methionine in codon 108, and if MB-COMT has a valine instead of methionine in codon 158, this is associated with an increased thermal stability of the protein. It is assumed that carriers of such alleles have a more stable and therefore more active form of COMT and therefore dopamine is better broken down in them. Various studies have shown that the presence of the valine variant is associated with an increased risk of schizophrenia. This finding would fit the hypofrontality thesis of schizophrenia. However, the results of the association studies on COMT / schizophrenia are very contradictory.
- The gene for RSG4 is on chromosome 1q22. It is a negative regulator of G-protein coupled receptors. RGS4 is regulated by dopaminergic activity and in turn regulates the activity of serotoninergic and glutamergic neurons. It interacts with ErbB3, which is a receptor on NRG1.
A summary of the data is given in the following table:
| author | Dysbindin | Neuregulin | DAOA | DISC 1 | COMT | RGS 4 |
|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
Of all the candidate genes mentioned, dysbindin and NRG1 are considered to be the most promising genes. However, numerous other candidates are named in the various reviews. They include the metabotropic glutamate receptor GRM-3 on chromosome 7q, glutamate decarboxylase 1 on chromosome 2q and a viral oncogene AKT1, which induces thymomas in mice. A selection of further findings can be added:
- Region 1. 4 in chromosome 1 was considered to be a good candidate , which was found due to a partial trisomy 5 in a family with two schizophrenic patients.
- In a number of families with schizophrenic patients, a partial translocation of chromosome 11 was found near the gene region in which genes for the D2 receptor , tyrosine kinases and NCAM (neuronal cell adhesion molecule) were found.
- There is a questionable association of a polymorphism of the 5-HT 2A receptor with a preferential response to clozapine .
- Markers on chromosome 18p may relate to schizophrenic and affective psychosis.
Because of the large number of markers that have been found for schizophrenia on practically all chromosomes with the exception of number 23, more and more scientists are now doubting whether the previous research strategies for the genetics of schizophrenia will lead to success. The American geneticist Lynn DeLisi has therefore suggested that we no longer look for disease-associated mutations. She suggested examining the methylation status of the X chromosome in patients with Klinefelter syndrome and schizophrenia. The idea is that an incorrect inactivation of certain genes on the X chromosome contributes to the risk of developing schizophrenia. To this end, DeLisi and others studied the methylation status of X-linked genes that are unique to humans.
Neurochemistry and Neuropharmacology
It seems reasonable to assume similar neuronal causes for the psychopathological phenomena as for the normal psychological functions. However, due to the variety of symptoms of schizophrenia, it cannot be assumed that there is a specific neurochemical disorder pattern. Since the discovery of neuroleptics , a large area of scientific research into the causes of schizophrenia has focused on the question of the importance of the dopaminergic system in the human brain for the development of schizophrenia. In recent years, other transmitter systems have also been increasingly examined. One of the reasons for this is the discovery of so-called atypical neuroleptics.
Dopamine
The principle of action of the classic neuroleptics is to block the dopamine receptors in the brain. This leads to a changed activity of the nerve cell clusters supplied by the dopaminergic system.
Anatomy of the dopaminergic systems
There are four dopaminergic systems in the human brain. The nigro striatal system is a connection of dopaminergic neurons from the brain stem to the basal ganglia . Disorders of the nigrostriatal system lead to movement disorders in Parkinson's disease . Similar symptoms occur when taking neuroleptics. The tuberoinfundibular system consists of dopaminergic neurons that regulate prolactin secretion. The use of neuroleptics often leads to an increase in serum prolactin and corresponding side effects. The mesolimbic system is responsible for the regulation of affects . The mesofrontocortical and mesohippocampal systems are held responsible for processes in the area of cognition and memory .
Dopamine and dopamine receptors
There are two different families of dopamine receptors, identified by the abbreviations D1 and D2. The D1 family contains the two subtypes D1 and D5. The D2 family contains the three subtypes D2, D3 and D4. The D2 receptors are mainly responsible for the antipsychotic effects of neuroleptics. Measurements of dopamine concentrations in people with schizophrenia gave very contradicting results. Presumably, the excess dopamine that causes schizophrenia does not contribute significantly to the measurable concentrations. The dopamine metabolite homovanillic acid can be measured in the CSF. Its concentration correlates with the consumption of neuroleptics. In schizophrenic patients, post-mortem studies found an increased number of D2 receptors in the brain, which, according to current opinion, is caused by the use of neuroleptics. Numerous studies dealt with the detection of radioactively labeled neuroleptics bound to D2 receptors in patients and test persons. The results of these studies allow the following conclusions: at the usual doses of typical neuroleptics, 70-80% of the receptors are blocked. There are no differences between responders and non-responders. Classic neuroleptics also block D1 receptors, while atypical ones also block serotonin receptors.
The dopamine hypothesis of schizophrenia
Over forty years ago, Carlson and Snyder hypothesized that psychotic symptoms are caused by an excess of dopamine. Blocking the dopamine receptors will then, as in the case of neuroleptics, alleviate psychotic symptoms. On the other hand, taking amphetamines can have a euphoric effect and, if taken for a long time, trigger psychoses. Amphetamine causes dopamine to be released and inhibits its inactivation. Amphetamine psychoses respond very quickly to the administration of neuroleptics. These observations support the dopamine hypothesis. The following problems of the dopamine hypothesis were discussed:
- Schizophrenic negative symptoms are aggravated by dopamine blockade. For this reason, Tim Crow postulated the existence of two different forms of schizophrenia (type I and type II).
- The clinical effect of neuroleptics does not set in as quickly as the pharmacological effect.
After a sufficient dose of a neuroleptic has been administered, all dopamine receptors are occupied within two hours at the latest. The antipsychotic effect often only sets in when a neuroleptic is taken for days or even weeks. Therefore, the antipsychotic mechanism of action is not assumed to be the receptor blockade itself, but rather the delayed depolarization block .
Glutamate
A glutamate hypothesis of schizophrenia has also been discussed for over 15 years . A strong argument for this hypothesis is the existence of a phenomenon analogous to amphetamine psychosis, glutamate psychosis caused by phencyclidine (PCP). The psychosis-inducing effect of phencyclidine has long been known. In particular, the L isomer of the PCP derivative ketamine , which was used in veterinary medicine and earlier also in paediatrics for anesthesia , can trigger acute psychoses. PCP can trigger not only positive but also negative symptoms in healthy test subjects. PCP psychosis is therefore an ideal model for schizophrenia.
Regarding the neuroanatomy of the glutamergic neurons , it should be noted that glutamate is the most important neurotransmitter of the cortical neurons . Eight glutamergic receptors have been identified so far. They are divided into two groups: three ionotropic and five metabotropic receptors. Of all the glutamate receptors, the NMDA receptor is psychiatrically the most interesting. It has been studied intensively for over 20 years. For an overview, compare:
Serotonin (5-HT)
The serotonergic system has an elementary influence on perception and sensation. The hallucinogenesis of “classic” psychedelic hallucinogens , such as mescaline , can be traced back to serotonergic mechanisms. The 5-HT 2A receptor is of central importance . Drugs that preferentially inhibit the 5-HT 2A receptor (MDL 100907), for example, have an antipsychotic effect. Other serotonin receptors are under discussion as to being involved in the overall mind-altering effects of some hallucinogens, including 5-HT 1A , 5-HT 5A , 5-HT 7 . Nevertheless, it is difficult to derive a conclusive explanatory model for schizophrenia from these findings. Because schizophrenic patients experience the psychoses caused by serotonergic hallucinogens differently than the symptoms of illness with which they are familiar. (H. Leuner) This limits the value of “ LSD ” psychosis as a model for schizophrenia research. Osmond and Symythies set up the transmethylation hypothesis of schizophrenia in 1952. This says that the body's own substances are converted into substances that trigger psychosis, similar to LSD. This hypothesis has not been proven.
Other neurochemical findings
Preliminary studies have shown in post-mortem examinations an increase in the proportion of dopamine receptor dimers in schizophrenia.
Morphological Findings
Beginnings
Neuropathological examinations in people suffering from schizophrenia date back to the beginning of brain research. In the 1890s Emil Kraepelin founded a neuroanatomical laboratory at the University of Munich . His students Alois Alzheimer , Robert Gaupp and Franz Nissl began their studies there on the neuropathology of schizophrenia.
Alzheimer published one of the first papers on this topic in 1897. Numerous studies on this topic had been published by the middle of the 20th century, but they could not show any uniform results. Therefore, hardly any publications in this area have been published over a period of more than 30 years.
Non-functional imaging
Pneumoencephalography
The beginning of modern research on the morphological changes in schizophrenia was formed by the pneumencephalographic examinations carried out by Gerd Huber in the 1950s. In doing so, Huber discovered the ventricular asymmetry in schizophrenics. In pneumencephalography, the CSF is largely exchanged for air and conventional skull x-rays are made. The air in the inner and outer cavities of the brain creates a contrasting effect that allows the outlines of the brain and, depending on the patient's position, parts of the ventricular system to appear three-dimensional. In the era before computed tomography, this procedure was used, among other things, to diagnose brain tumors and to plan stereotactic operations.
CCT and MRI
Since the invention of computed tomography and the first CT study in schizophrenic patients by Johnstone, over 200 computed tomography and magnetic resonance tomography studies have been performed in these patients. These studies have shown that people with schizophrenia have markedly enlarged left lateral ventricles. The findings indicate that there are no other subgroups with a special type of ventricular dilation among people with schizophrenia with ventricular asymmetry. There is also no clear correlation between the ventricle width and a specific symptom. In addition, this phenomenon is not schizophrenia-specific, it is also found in patients with mood disorders. The ventricular dilation appears to be genetically determined. At least there is no correlation in the patients with any other examined characteristic such as age, gender, treatment, social group etc. However, the ventricular dilation is also found in the close relatives of the patients. So far there are no results from prospective studies on the question of the risk of disease in ventricular dilatation. The cause of the phenomenon is believed to be a reduction in the number of cells that are adjacent to the ventricular spaces. The ventricular dilation is partly due to volume reductions in the area of the hippocampus. To overview:
Volumetric studies in schizophrenia
- Total volume
Up to 2001 there were 23 studies in which changes in the total volume of the brain of schizophrenic patients were examined. It was found that in the majority of cases there were no significant changes. Some of the patients showed a decrease in brain volume.
- Volume changes in gray and white matter.
Studies of changes in the volume of gray and white matter have shown significant changes. In the overwhelming majority of the cases examined in a total of 20 studies up to the year 2000, there was a reduction in the volume of gray matter, with the measured volumes of white matter remaining unchanged. Individual studies have shown an effect of volume reduction on the action of neuroleptics.
- Ventricular volume
Lateral ventricular enlargement in schizophrenia is the most commonly replicated finding. This could be proven in a compilation of 28 studies from the years 1994 to 2000 in over 95% of the cases. The causes of the ventricular asymmetry are not exactly known. A general loss of brain tissue could be demonstrated in individual studies. In a large comparative study to predict nerve cell loss based on the data of the ventricle volume and the CSF volume above the brain surface, it was shown that an enlarged ventricle volume in the case of schizophrenia could be accompanied by a reduced volume of gray matter. This is remarkable because an enlarged ventricle volume is usually due to a volume reduction of the basal ganglia, which is not the case with schizophrenia. In a methodologically complex MRT study, it could be shown that there are no differences between first-time and chronically ill patients with schizophrenia in terms of enlarged ventricular volume, which is compatible with the hypothesis of a neuronal developmental disorder.
- Cortical structures
- Temporal lobe
Of all the cortical structures, changes in the area of the temporal lobe have been studied best in the case of schizophrenia. The most certain finding is a volume reduction in the area of the left temporal lobe in which the Wernicke language center is located (planum temporale / gyrus temporalis superior). These findings could be confirmed by imaging procedures and post-mortem studies. The changes correlate with psychopathological findings. The medial section of the temporal lobe is the subject of particular interest because it is where there are regions where abnormalities were found in post-mortem studies. In 1986, Jakob and Beckmann found abnormal neuron populations in the enthorinal cortex, and in the majority of the studies that were carried out, a volume reduction was found in the hippocampus. In addition, the post-mortem studies showed a reduced neuron size, which indicates a disturbed connectivity of the affected neurons.
- Frontal lobes
The best-studied findings regarding the frontal lobes in schizophrenia relate to metabolic hypofrontality. With regard to morphological changes, it is noteworthy that the normal asymmetry of the frontal lobe (right> left) is eliminated in schizophrenia. Since the frontal lobe is divided into many subunits, studies have been carried out on individual sections of this brain region in schizophrenia. It was found that there is a decrease in volume in schizophrenia, especially in the area of the dorsolateral prefrontal cortex. In individual studies, a disorder of gyrification was also found in this area .
- Parietal lobes
Studies on the parietal lobes in schizophrenia have so far not shown any characteristic changes. A decrease in gray matter was found in individual subregions.
- Subcortical structures
- Basal ganglia
The so-called basal ganglia are the location of neural connections between different cortical areas (parietal and prefrontal) and are part of a so-called neural loop from the cortex via the BG and thalamus back to the cortex. These connection structures provide for the selection and initiation of voluntary actions. In 14 studies from 1994 to 2000, structural changes were found in 80% of the cases: In patients with schizophrenia who were treated with neuroleptics, the BG increased in volume and in neuroleptic-naive patients a decrease in volume.
- Thalamus
The thalamus is a kind of filter for sensory information between the cortex and the limbic system. The majority of the performed MRI studies showed a volume reduction of the thalamus in schizophrenia. On the one hand, it was shown that the volume reduction is bilateral and that it is associated with reduced perfusion.
- Cerebellum
The cerebellum exercises control functions of fine motor skills. There is also evidence of its involvement in cognitive processes. Nancy Andreasen wrote the theory of "cognitive dysmetria". It states that the cerebellum is involved in the symptoms of schizophrenia through connections with the prefrontal cortex and thalamus. The investigations carried out so far are based on the finding of a reduction in volume of the cerebellar worm. However, the data situation on this finding has so far been rather limited.
Functional imaging
PET and SPECT examinations
The best-known finding from functional imaging examinations using PET and SPECT is the phenomenon of hypofrontality in schizophrenic patients, discovered in 1971. Franzen et al . Observed in their pioneering study a reduction in the frontal cerebral blood flow . The frontal underperfusion is 1–8% in all studies carried out since then. The phenomenon was very soon linked to the assumption that in schizophrenics the frontal cortex shows less dopaminergic activation. It can be assumed that a kind of counter-regulatory effort by the frontal neurons leads to dopaminergic overdrive. The relative excess dopamine in the limbic system and in other cortical regions caused by hypofrontality could then lead to the psychotic symptoms of schizophrenia.
FMRT and MRS studies
The reduced activation of the dorsolateral prefrontal cortex was also confirmed with the help of functional magnetic resonance imaging . The evidence that patients who hear voices show activation in the primary acoustic cortex was particularly impressive. Using phosphorus-31 M agnet- R esonanz- S spectroscopy was also found a reduced energy expenditure in the frontal brain of schizophrenic patients. The neuron-specific substances ( N-acetyl aspartate ) and the marker for degenerative processes choline can be detected using hydrogen spectroscopy . In schizophrenic patients, a reduction in the NAA was consistently found in the hippocampus, with unchanged values for choline. This means that the reduced activation of the hippocampus in people with schizophrenia is not due to degenerative processes. To overview:
Imaging and cognitive performance
The following table provides an overview of the findings on the localization of cognitive deficits in patients with schizophrenia.
| Cognitive domain | Description of cognitive performance |
Brain region with impaired signal change |
|---|---|---|
|
|
|
Neuropathological Findings
Due to the radiological findings on ventricular asymmetry by Huber and Johnstone, there was a renaissance in neuropathological investigations of schizophrenia in the early 1980s.
Limbic system
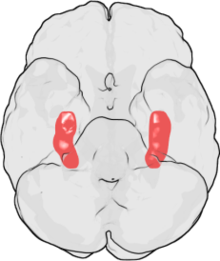
The work of Bogerts marks the beginning of modern neuropathological investigations into schizophrenia. Since 1984, his working group has shown in various studies that there is a reduction in volume in the area of the hippocampus and amygdala in people with schizophrenia . Volume reductions in the thalamus area have also been observed, but the findings are not as significant. Changes have also been described in the area of the cingulate gyrus .
Entorhinal area
The entorhinal area has been extensively studied in schizophrenic patients and the findings are controversial. The working group around Jakob and Beckmann documented the findings well, others could not reproduce them. For an overview cf. In particular, it involves the observation of abnormally rotated neurons. The main problem with these studies is the small number of cases: In total, post-mortem studies have been carried out for this purpose in hardly more than two dozen patients.
Migration disorders
Changes similar to those in the entorhinal area were also found in some cases in the hippocampus. It is believed that the cause of these rotated neurons are migration disorders. Since such changes were also found in the schizophrenic victims of the atomic bombing in Hiroshima and Nagasaki, studies were carried out with the question of whether people with schizophrenia have more mutations in the area of DNA repair systems. These studies did not give a positive result. However, observations that later schizophrenic patients showed above-average neurological deficits (motor clumsiness or atavistic reflexes such as the finger splay phenomenon) fit in with the assumption of a genetic or early acquired migration disorder .
Volume reduction
Neuroradiological studies have shown that there are morphological changes in patients with schizophrenia. The cause of this change has been intensively researched for around 20 years. However, the findings cannot yet be clearly interpreted. One reason for this is the small number of studies. An overview from 2002 lists only 70 post-mortem examinations. Very few findings are clearly replicated, and only a few studies are methodologically comparable. For example, although the reduction in volume of the hippocampus and amygdala in schizophrenic patients has now been proven beyond doubt by imaging methods, there are no replicated neuropathological findings on volume reduction or nerve cell loss in the hippocampus. So the cause of this change is so far unclear.
causes
Although the data situation for the neuropathological examinations appears confusing, there are clear findings. In none of the investigations was there any evidence of changes that would be expected in classic degenerative brain processes. The morphological changes in schizophrenia are therefore not comparable with those in Alzheimer's disease, multiple sclerosis or Huntington's chorea. For an overview cf.
Other organic factors
Birth complications
In the early 1960s, Mednick and Schulsinger claimed that birth complications were a risk factor for schizophrenia. However, the studies carried out so far have shown that the risk of disease due to complications in childbirth increases by a maximum of 1%. Occasionally the assumption has been made that, conversely, the genetic disposition to schizophrenia is associated with maturation disorders and then possibly leads to secondary birth complications.
Infection and immune hypotheses
The first infection and immune hypotheses go back to Wagner-Jauregg . He received the Nobel Prize for Medicine for his malaria experiments (vaccine malaria) in psychotic patients with syphilis. There is no solid data to support these hypotheses. Neither have any indications of the presence of an inflammatory disease of the CNS been found in schizophrenic patients in the course of post-mortem examinations, nor are there consistent results in examinations for specific antibodies against neurotropic viruses or foreign DNA. Of course, the negative findings do not rule out the possibility of infections with pathogens that integrate into the genome or that only multiply intracellularly ( Borrelia ). There are, however, epidemiological data in favor of the infection hypothesis: retrospectively, it was found that if the mother of a later patient fell ill with a viral flu during flu epidemics in the second trimester, the risk of the unborn child of developing schizophrenia later on increased . There is also a predominance of winter births from schizophrenic patients in the northern hemisphere, which is associated with an increased risk of developing viral infections in the mother during the second trimester.
Recent research points towards the unspecific activation of certain parts of the immune system. There are also indications of changes in the tryptophan and kynurenine metabolism. Disturbances of the kynurenine metabolism in humans with increased L-kynurenine or metabolite values have been described for schizophrenia and other psychiatric diseases. Typically, there is an accumulation of kynurenine and a shift in tryptophan metabolism towards kynurenic acid, anthranilic acid and their other metabolic products. A frequent constellation in various neuropsychiatric diseases is a simultaneous increased kynurenine / tryptophan ratio due to the accumulation of kynurenine before the next metabolic step, the hydroxylation to 3-hydroxykynurenine as a result of catalysis by kynurenine-3-monooxygenase (KMO).
Summary
If these findings are summarized, a surprisingly uniform picture emerges from a neurobiological point of view. Schizophrenia has a genetic component, although it is completely unclear what this genetic component looks like. The receptor / transmitter studies suggest that not one but several functional systems are involved in the development of schizophrenia. The morphological studies of schizophrenia show that there is a developmental or a low-grade degenerative component of the disease. Functional examinations give clear indications that the psychotic symptoms are related to malfunctions of the conventional functional systems of the brain (auditory cortex and voice hearing). Environmental factors certainly play a role in schizophrenia, but infection does not seem to be one of them.
See also
- Clinical schizophrenia concepts
- Symptoms and diagnosis of schizophrenia
- Subtyping of schizophrenia
- Onset and early course of schizophrenia
- Late schizophrenia
- Course of schizophrenia
- Therapy for schizophrenia
literature
- Matcheri S. Keshavan, Rajiv Tandon, Nash N. Boutros, Henry A. Nasrallah: Schizophrenia. “Just the facts”: What we know in 2008 . Part 3: Neurobiology . In: Schizophrenia Research , 106 (2008), pp. 89-107.
- Christopher A. Ross, Russell L. Margolis, Sarah AJ Reading, Mikhail Pletnikov, Joseph T. Coyle : Neurobiology of Schizophrenia . In: Neuron , 52 (2006), pp. 139-153.
Individual evidence
- ↑ J. Kornhuber, J. Wiltfang, S. Bleich: The aetiopathogenesis of schizophrenias . In: Pharmacopsychiatry , 37 (2004), Suppl. 2, pp. 103-112.
- ^ Hans-Walter Schmuhl (Ed.): Race research at Kaiser Wilhelm Institutes before and after 1933 . Wallstein-Verlag, Göttingen 2003, ISBN 3-89244-471-4 .
- ↑ Volker Roelcke : Program and practice of psychiatric genetics at the German Research Institute for Psychiatry under Ernst Rüdin . In: Schmuhl: Rassenforschung .
- ↑ Ernst Rüdin: On inheritance and the emergence of dementia praecox . Berlin 1916.
- ↑ E. Salter, V. Cowie (Ed.): Genetics of Mental Disorders . Oxford 1970.
- ↑ M. Fischer u. a. In: Br. J. Psychiatry , 115 (1969), pp. 981-990, PMID 5387002
- ↑ S. Onstad et al. a. In: Acta Psychiatr. Scand. , 83 (1991), pp. 395-401, PMID 1853734
- ↑ Seymour S. Kety et al. a .: Mental illness in the biological and adoptive relatives of schizophrenic adoptees. Replication of the Copenhagen Study in the rest of Denmark . In: Arch Gen Psychiatry 51 (1994), pp. 442-455, PMID 8192547
- ^ II Gottesman, A. Shields: A critical review of recent adoption, twin and family studies of schizophrenia: behavioral genetics perspectives . In: Schizophrenia Bulletin , 2 (1976), pp. 360-401, PMID 1034336 .
- ↑ CM Lewis et al. a .: Genome-scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia . In: American Journal of human genetics , 2003, 73, 1, pp. 34-48, PMID 12802786 .
- ↑ G. Kirov et al. a .: Finding Schizophrenia Genes . In: J. Clini. Invest. , 2005 115 (6), pp. 1440-1448, PMID 15931379 .
- ↑ AS Bassett: Chromosomal aberrations and schizophrenia. Autosomes . In: British Journal of Psychiatry , 161 (1992), pp. 323-334, PMID 1393302 .
- ↑ G. Silberberg, A. Darvasi, R. Pinkas Kramarski, R. Navon: The involvement of ErbB4 with schizophrenia: association and expression studies . In: Am J Med Genet B Neuropsychiatr Genet. , 2006 Mar 5; 141 (2), pp. 142-148, PMID 16402353
- ↑ DR Weinberger: Genetic mechanisms of psychosis: in vivo and postmortem genomics . In: Clin Ther. , 2005; 27 Suppl A: S8-15 , PMID 16198200
- ^ N. Norton, HJ Williams, MJ Owen: An update on the genetics of schizophrenia . In: Curr Opin Psychiatry , 2006 Mar; 19 (2), pp. 158-164, PMID 16612196
- ↑ George Kirov, Michael C. O'Donovan, Michael J. Owen: Finding schizophrenia genes . In: J Clin Invest. , 2005 June 1; 115 (6), pp. 1440-1448, PMID 15931379
- ↑ MJ Owen, N. Craddock, MC O'Donovan: Schizophrenia: genes at last? In: Trends Genet. 2005 Sep; 21 (9), pp. 518-525, PMID 16009449
- ^ PJ Harrison, DR Weinberger: Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence . In: Mol Psychiatry , 2005 Jan; 10 (1), pp. 40-68, PMID 15263907
- ↑ BH Shirts, V. Nimgaonkar: The genes for schizophrenia: finally a breakthrough? In: Curr Psychiatry Rep. , 2004 Aug; 6 (4), pp. 303-312, PMID 15260947
- ↑ S. Mah u. a .: Identification of the semaphorin receptor PLXNA2 as a candidate for susceptibility to schizophrenia . In: Mol Psychiatry , (2006) 11, pp. 471-478, PMID 16402134 .
- ↑ H. Häfner (Ed.): Risk and protective factors in schizophrenia. Towards a conceptual model of the disease process . Steinkopff, Darmstadt 2002.
- ^ P. Propping : Psychiatric Genetics. Findings and concepts . Springer, Berlin 1989.
- ↑ NL Ross, R. Wadekar, A. Lopes, A. Dagnall, J. Close, LE Delisi, TJ Crow: methylation of two Homo sapiens-specific XY homologous genes in Klinefelter's syndrome (XXY) . In: Am J Med Genet B Neuropsychiatr Genet. , 2006 Jul 5; 141 (5), pp. 544-548, PMID 16741946
- ^ A. Carlson: Antipsychotic drugs, neurotransmitters and schizophrenia . In: American Journal of Psychiatry , 135, 1978, pp. 164-173.
- ↑ SH Snyder: The dopamine hypothesis of schizophrenia: focus on the dopamine receptor . In: Am J Psychiatry . 1976 Feb; 133 (2), pp. 197-202, PMID 1251927
- ^ AS Horn, SH Snyder: Chlorpromazine and dopamine: conformational similarities that correlate with the antischizophrenic activity of phenothiazine drugs . In: Proc Natl Acad Sci USA , 1971 Oct, 68 (10), pp. 2325-2328, PMID 5289865 .
- ^ SH Snyder: Catecholamines in the brain as mediators of amphetamine psychosis . In: Arch Gen Psychiatry , 1972 Aug; 27 (2), pp. 169-179, PMID 4339577 .
- ↑ SH Snyder: Amphetamine psychosis: a “model” schizophrenia mediated by catecholamines . In: Am J Psychiatry. , 1973 Jan, 130 (1), pp. 61-67, PMID 4345465 .
- ^ TJ Crow: Molecular pathology of schizophrenia: more than one disease process? In: Br Med J , 280, 1980, pp. 66-68, PMID 6101544 .
- ↑ N. Yu, KR Tucker, ES Levitan, PD Shepard, CC Canavier: Implications of cellular models of dopamine neurons for schizophrenia. In: Progress in molecular biology and translational science. Volume 123, 2014, pp. 53-82, doi : 10.1016 / B978-0-12-397897-4.00011-5 , PMID 24560140 , PMC 4351765 (free full text) (review).
- ↑ RD Paz, S Tardito, M Atzori, KY. Tseng: Glutamatergic dysfunction in schizophrenia: from basic neuroscience to clinical psychopharmacology . In: Eur Neuropsychopharmacol . 2008 Nov; 18 (11): 773-86. Epub 2008 Jul 23rd Review, PMID 18650071
- ↑ EH Wong, JA Kemp, T Priestley, AR Knight, GN Woodruff, LL. Iversen: The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist . In: Proc Natl Acad Sci USA . 1986 Sep; 83 (18), pp. 7104-7108, PMID 3529096
- ↑ DC Goff, JT Coyle: The emerging role of glutamate in the pathophysiology and treatment of schizophrenia . In: American Journal of Psychiatry , 158, 2001, pp. 1367-1377, PMID 11532718 .
- ^ DE Nichols: Hallucinogens . In: Pharmacol Ther , 101, 2004, pp. 131-181, PMID 14761703
- ↑ NJ Penington et al. a .: Effects of LSD on Calcium-currents in central 5-HT-containing neurons: 5-HT (1A) receptors may play a role in hallucinogenesis . In: J. Pharmacol. Exp. Ther. , 269, 1994, pp. 1160-1165, PMID 8014859 .
- ↑ H. Osmond, J. Smythies: Schizophrenia: new approach . In: J. Mental. Sci. , 98, 1952, pp. 309-322, PMID 14917992 .
- ↑ M Wang u. a .: Schizophrenia, amphetamine-induced sensitized state and acute amphetamine exposure all show a common alteration: increased dopamine D2 receptor dimerization . In: Mol Brain , 2010, 3, p. 25, PMID 20813060
- ^ A. Alzheimer: Contributions to the pathological anatomy of the cerebral cortex and to the anatomical basis of some psychoses . In: Mschr. Psychiat. Neurol . 2, pp. 82-120, 1897.
- ^ G Peters: Neuropathology and Psychiatry . In: HW Gruhle, (Ed.): Psychiatry of the present . Springer, Berlin 1967, Volume I, pp. 298-305.
- ↑ G. Huber: Chronic Schizophrenia. Synopsis of clinical and radiological examinations on defective schizophrenic institutional patients . Hüthig, Heidelberg, 1961.
- ↑ Gergely Klinda: On the history of pneumencephalography . Dissertation, Charité Berlin 2010
- ↑ EC Johnstone et al. a .: Cerebral ventricular size and cognitive impairement in chronic schizophrenia . In: Lancet , 2, 1976, pp. 924-926, PMID 62160 .
- ↑ JD Van Horn et al. a .: Ventricular enlargement in schizophrenia. A meta-analysis of studies of the ventricle: brainratio (VBR) . In: Br. J. Psychiatry , 160, 1992, pp. 687-697, PMID 1534268 .
- ↑ A. Schmitt et al. a .: Current overview of structural magnetic resonance imaging in schizophrenia . In: Advances in Neurology Psychiatry , 2001, 69, pp. 105–115, PMID 11305121
- ↑ RE Gur u. a .: Reduced gray matter volume in schizophrenia . In: Arch Gen Psychiatry , 1999, 56, pp. 905-911, PMID 10530632
- ↑ RB Zipursky et al. a .: Cerebral gray matter volume deficits in first episode psychosis . In: Arch Gen Psychiatry , 1998, 55, pp. 540-546, PMID 9633673
- ↑ C. Arango et al. a .: The relationship of clozapin and haloperidol treatment response to prefrontal, hippocampal and caudate brain volumes . In: Neuroreport , 2003, 14, pp. 2025-2029, PMID 12900303
- ↑ DF Braus: Schizophrenia . Schattauer Verlag, 2005, ISBN 3-7945-2316-4 , p. 116.
- ↑ PJ. Harrison: Brains at risk of schizophrenia . In: Lancet , 1999, 353, pp. 3-4, PMID 10023939
- ↑ LL Symonds et al. a .: Does an increase in sulcal or ventricular fluid predict where brain tissue is lost? In: J Neuroimaging , 1999, 9, pp. 201-209, PMID 10540599 .
- ↑ DF Braus: Schizophrenia . Schattauer Verlag, 2005, ISBN 3-7945-2316-4 , p. 118 f.
- ^ IC Wright et al. a .: Meta-analysis of regional brain volumes in schizophrenia . In: Am J Psychiatry , 2000, 157, pp. 16-25, PMID 10618008
- ↑ a b RW. McCarley et al. a .: MRI anatomy of schizophrenia . In: Biol Psychiatry , 1999, 45, pp. 1099-1119, PMID 10331102
- ^ RP Rajarethinam et al. a .: Superior temporal gyrus in schizophrenia: a volumetric MRI study . In: Schizophrenia Research , 2000; 41, pp. 303-312, PMID 10708339
- ↑ P. Falkai et al. a .: Disturbed planum temporale asymmetry in schizophrenia . In: Schizophrenia Research , 1995; 14, pp. 161-176, PMID 7710997
- ↑ C. Levitan et al. a .: Superior temporal gyral volumes and laterality correlates of auditory hallucinations in schizophrenia . In: Biol Psychiatry , 1999, 46, pp. 955-962, PMID 10509178
- ^ H Jakob, H. Beckmann: Prenatal developmental disturbances in the limbic allocortex in schizophrenics . In: J Neural Transm , 1986, 65, pp. 303-326, PMID 3711886
- ↑ SE Arnold u. a .: Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical-hippocampal interactions . In: Am J Psychiatry , 1995, 152, pp. 738-748, PMID 7726314
- ↑ K Henke u. a .: Human hippocampus associates information in memory . In: PNAS , 1999; 96, pp. 5884-5889, PMID 10318979
- ↑ DW Zaidel u. a .: Size, shape and orientation of neurons in the left and right hippocampal investigation of normal asymmetries and alterations in schizophrenia . In: Am J Psychiatry , 1997, PMID 9167509
- ↑ CS Carter et al. a .: Functional hypofrontality and working memory dysfunction in schizophrenia . In: Am J Psychiatry , 1998; 155, pp. 1285-1287, PMID 9734557
- ↑ J Schröder u. a .: Cerebral metabolic activity correlates of subsyndroms in chronic schizophrenia . In: Schizophrenia Research , 1996; 19, pp. 41-53, PMID 9147495
- ↑ RM pictures u. a .: Absence of regional hemispheric volume asymmetries in first episode schizophrenia . In: Am J Psychiatry , 1994; 151, pp. 1437-1447, PMID 8092337
- ↑ TE Schlaepfer u. a .: Decreased regional cortical gray matter volume in schizophrenia . In: Am J Psychiatry , 1994; 151, pp. 842-848, PMID 8184992
- ↑ K Vogeley et al. a .: Right frontal hypergyria differentiation in affected and unaffected siblings from families multiply affected with schizophrenia: a morphometric MRI study . In: Am J Psychiatry , 2001; 158, pp. 494-496, PMID 11229998
- ↑ S. Heckers: Neuropathology of Schizophrenia . In: Schizophrenia Bulletin , 1997, 23, pp. 403-421, PMID 9327506
- ↑ MH Chakos u. a .: Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine . In: Lancet , 1995; 345, pp. 356-357, PMID 7853978
- ↑ AM Elkashef u. a .: Basal ganglia pathology in schizophrenia and tardive dyskinesia: an MRI quantitative study . In: Am J Psychiatry , 1995; 151, pp. 752-755, PMID 7909412
- ^ A Carlsson et al. a .: Neurotransmitter aberrations in schizophrenia . In: Life Sci , 1997; 61, pp. 75-94, PMID 9217267
- ↑ C Gaser u. a .: Detecting structural changes in whole brain based on nonlinear deformations-application to schizophrenia research . In: Neuroimage , 1999; 10, pp. 107-113, PMID 10417245
- ↑ MS Buchsbaum u. a .: PET and MRI of the thalamus in never-medicated patients with schizophrenia . In: Am J Psychiatry , 1996; 153, pp. 191-199, PMID 8561198
- ↑ NC Andreasen u. a .: Defining the phenotype of schizophrenia: cognitive dysmetria and is neural mechanisms . In: Biol Psychiatry , 1999; 46, pp. 908-920, PMID 10509174
- ↑ G end u. a .: Further evidence for altered cerebellar neuronal integrity in schizophrenia . In: Am J Psychiatry , 2005; 162, pp. 790-792, PMID 15800155
- ^ G. Franzen, DH Ingvar: Absence of activation in frontal structures during psychological testing of chronic schizophrenics . In: J Neurol Neurosurg Psychiatry , 38 (1975) pp. 1027-1032, PMID 1202164 .
- ↑ DR Weinberger u. a .: fMRI applications in schizophrenia research . In: Neuroimage , 4, 1996, pp. 118-126, PMID 9345536 .
- ↑ T. Dierks et al. a .: Activation of Heschls gyrus during auditory hallucinations . In: Neuron , 22, 1999, pp. 615-621, PMID 10197540 .
- ↑ PK McGuire et al. a .: Increased blood flow in Brocas Area during auditory hallicinations in schizophrenia . In: Lancet , 342, 1993, pp. 703-705, PMID 8103821 .
- ↑ a b T. Kircher u. a .: Functional imaging using the example of schizophrenia . In: Deutsches Ärzteblatt , 101, 2004, pp. A1975–1980.
- ↑ B. Bogerts: Neuropathology of Schizophrenia . In: Fortschr. Neurol. Psychiat , 52, pp. 428-437, 1984, PMID 6519627
- ^ H. Jakob, H. Beckmann: Prenatal developmental disturbances in the limbic allocortex in schizophrenics . In: J. Neural Transm. , 1986, 65, 303, PMID 3711886
- ↑ P. Falkai et al. a .: Entorhinal cortex pre-alpha cell clusters in schizophrenia: quantitative evidence of a developmental abnormality . In: Biol Psychiatry , 47, 2000, pp. 937-943, PMID 10838061
- ↑ P. Falkai et al. a .: Neuropathology . In: MG Gelder (Ed.): New Oxford Textbokk of Psychiatry . 2000.
- ↑ P. Falkai et al. a .: No evidence for astrogliosis in brains of schizophrenic patients. A post-mortem study . In: Neuropathol Appl Neurobiol . 25, pp. 48-53, 1999, PMID 10194775
- ↑ SA Mednick, F. Schulsinger: Studies of children at high risk for schizophrenia , pp 247-293. In: SR Dean (Ed.): Schizophrenia . MSS Information, New York 1973, ISBN 0-8422-7115-5 .
- ↑ TF McNeil: Review Article. Obstetric complications in schizophrenic parents . In: Schizophr. Res. , 5 (1991) pp. 89-101, PMID 1931811 .
- ^ Norbert Müller, Markus J. Schwarz: Immune System and Schizophrenia . In: Curr Immunol Rev. , 2010, 6, pp. 213-220.
- ↑ a b Ikwunga Wonodi, O. Colin Stine, Korrapati V. Sathyasaikumar et al .: Downregulated Kynurenine 3-Monooxygenase Gene Expression and Enzyme Activity in Schizophrenia and Genetic Association With Schizophrenia Endophenotypes . In: Arch Gen Psychiatry . 2011; 68, pp. 665-674.
- ↑ a b Maria Holtze, Peter Saetre, Göran Engberg, et al .: Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls . In: J Psychiatry Neurosci . 2012; 37, pp. 53-57.
- ↑ a b PJ Hoekstra, GM Anderson, PW Troost: Plasma kynurenine and related measures in tic disorder patients . In: Eur Child Adolesc Psychiatry . 2007; 16 Suppl 1, pp. 71-77.
- ^ Brian M. Campbell, Erik Charych, Anna W. Lee, Thomas Möller: Kynurenines in CNS disease: regulation by inflammatory cytokines . In: Frontiers in Neuroscience. Neuroendocrine Science 2014, Volume 8, Article 12.
- ↑ Serdar M. Dursun, Gillian Farrar, Sheila L. Handley, et al .: Elevated plasma kynurenine in Tourette syndrome . In: Molecular and Chemical Neuropathology , 1994, 21, pp. 55-60
- ↑ H. Rickards, SM Dursuna, G. Farrar: Increased plasma kynurenine and its relationship to neopterin and tryptophan in Tourette's syndrome . In: Psychological Medicine , 26, 1996, pp. 857-862
- ↑ Erik Kwidzinski: Involvement of indolamine 2, 3-dioxygenase (IDO) in immune regulation of the central nervous system . Dissertation, Charité Berlin, February 13, 2006, urn : nbn: de: kobv: 11-10059777
- ↑ K. Schroecksnadel, S. Kaser, G. Neurauter, et al .: Increased Degradation of Tryptophan in Blood of Patients with Rheumatoid Arthritis . In: The Journal of Rheumatology 2003; 30: 9
- ↑ M Maes, R Verkerk, S Bonaccorso et al .: Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation . In: Life Sci . 2002 Sep 6; 71 (16): 1837-48.