Niobium (V) chloride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Niobium (V) chloride | ||||||||||||||||||
| other names |
Niobium pentachloride |
||||||||||||||||||
| Molecular formula | NbCl 5 | ||||||||||||||||||
| Brief description |
yellow solid with a pungent odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 270.17 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.75 g cm −3 |
||||||||||||||||||
| Melting point |
208.3 ° C ( decomposition ) |
||||||||||||||||||
| boiling point |
248.2 ° C |
||||||||||||||||||
| solubility |
violent decomposition in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Niobium (V) chloride is a salt-like chemical compound consisting of the elements niobium and chlorine .
Extraction and presentation
Niobium (V) chloride can be obtained by reacting niobium with chlorine.
It is also possible to produce it by reacting niobium (V) oxide with thionyl chloride or carbon tetrachloride or hexachloropropene :
properties
It is a yellow solid with a pungent odor (formation of hydrogen chloride by hydrolysis ) that decomposes violently in water.
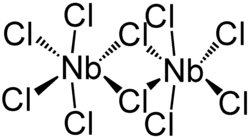
Niobium (V) chloride forms a dimeric structure in which each niobium atom is six-fold coordinated by chloro ligands . Two chlorine atoms act as bridging ligands . There are two crystalline polymorphs of niobium (V) chloride; a distorted octahedral structure is formed in both , in which the pseudo-axial chloro ligands are arranged at an angle of approx. 84 ° to the pseudo-equatorial plane.
use
Niobium (V) chloride is used as a Lewis acid in organic chemistry . For example, it is used in the activation of alkenes in the carbonyl-ene reaction .
Individual evidence
- ↑ a b c d e f g Entry on niobium (V) chloride in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1444.
- ^ WW Porterfield and SY Tyree, Jr .: Anhydrous metal chlorides . In: S. Young Tyree, Jr. (Ed.): Inorganic Syntheses . tape 9 . McGraw-Hill Book Company, Inc., 1967, p. 133-136 (English).
- ↑ W. Hoenle, H. G. v. Schnering: Crystal structure of niobium pentachloride . In: Zeitschrift für Kristallographie '' , 1990 , 191 , pp. 139–140 ( doi : 10.1524 / zkri.1990.191.1-2.139 )
- ↑ FA Cotton, PA Kibala, M. Matusz, RBW Sandor in: Structure of the Second Polymorph of Niobium Pentachlorid 1997 , Acta Crystallographica C47 , pp. 2435-2437 ( doi : 10.1107 / S0108270191000239 ).
- ↑ CKZ Andrade, OE Vercillo, JP Rodrigues, DP Silveira in J. Braz. Chem. Soc. 2004, 15, 6, 813-817.







