Octinoxate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula without stereochemistry in the side chain - 1: 1 mixture ( racemate ) | |||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Octinoxate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 18 H 26 O 3 | ||||||||||||||||||
| Brief description |
clear liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 290.40 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| Melting point |
−25 ° C |
||||||||||||||||||
| boiling point |
198-200 ° C |
||||||||||||||||||
| solubility |
insoluble in water |
||||||||||||||||||
| Refractive index |
1.545 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Octinoxate is a substance that is used as a UV filter in sunscreens.
Chemically, it is a mixture of at least two isomeric chemical compounds from the group of cinnamic acid esters . Octinoxat offers protection against UVB radiation with wavelengths of 280 to 320 nanometers . Octinoxate is controversial because of its effects as an endocrine disruptor on the estrogenic hormonal system .
Extraction and presentation
Octynoxate can be produced starting from p -bromanisole by a Heck reaction via the intermediate stage 4-methoxycinnamic acid and subsequent esterification of the carboxylic acid.
Stereochemistry
Octinoxate contains a stereocenter and a double bond. Sunlight can isomerize the ( E ) form to the ( Z ) form. The esterification to octynoxate requires 2-ethylhexanol, which can be prepared as a racematic mixture [a 1: 1 mixture of ( R ) - and ( S ) form]. Therefore octynoxate could consist of the following four stereoisomers:
| Isomers of octinoxate | ||
|---|---|---|
| ( R ) shapes | ( S ) shapes | |
| ( E ) shapes |
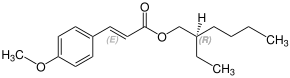
|

|
| ( Z ) shapes |
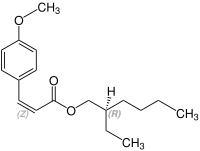
|
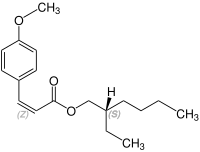
|
safety instructions
The effects of octinoxate on human health and the environment have been examined under REACH since 2016 as part of the substance assessment by Great Britain.
Trade names
- Tinosorb OMC
- Eusolex 2292
Admission
According to Regulation (EC) No. 1223/2009 on cosmetic products , octinoxate is permitted as a UV filter up to a maximum content of 10% (calculated as acid) in the ready-to-use preparation.
A law in the US state of Hawaii will prohibit the sale of sunscreens that contain octinoxate or oxybenzone from January 2021 . Both substances have a harmful effect on corals and the genetic make-up of fish. In the South Sea state of Palau , octinoxate has been banned in sun creams since January 2020.
Individual evidence
- ↑ Entry on ETHYLHEXYL METHOXYCINNAMATE in the CosIng database of the EU Commission, accessed on February 16, 2020.
- ↑ a b c d Datasheet Octyl methoxycinnamate at chemicalland21.com, accessed on October 31, 2017.
- ↑ a b c Data sheet 2-Ethylhexyl 4-methoxycinnamate, 98% from Sigma-Aldrich , accessed on February 17, 2014 ( PDF ).
- ↑ Data sheet at Clearsynth ( Memento from March 28, 2014 in the Internet Archive )
- ↑ smartskincare.com: Chemical UVB sunscreen / sunblock: octyl methoxycinnamate (octinoxate) , accessed October 31, 2017.
- ↑ ToxFox - The cosmetic check. Association for the Environment and Nature Conservation Germany
- ↑ Dirk Steinborn: Fundamentals of organometallic complex catalysis . 2nd Edition. Vieweg + Teubner, 2009, ISBN 978-3-8348-0581-2 ( page 251 in the Google book search).
- ↑ S. Pattanaargson, T. Munhapol, P. Hirunsupachot, P. Luangthongaram (eds.): Photoisomerization of octyl methoxycinnamate. In: Journal of Photochemistry and Photobiology A: Chemistry , Elsevier Verlag, Volume 161, No. 2-3, January 30, 2004, pp. 269-274.
- ↑ Process for the production of 2-ethylhexanol: DE 3530839 A1 , August 29, 1985; EP 0216151 B1 , August 20, 1986.
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 2-Ethylhexyl-trans-4-methoxycinnamate , accessed on October 31, 2017.
- ↑ Entry on octinoxate in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on October 31, 2017.
- ↑ REGULATION (EC) No. 1123/2009 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 30 November 2009 on cosmetic products (PDF), accessed on 14 March 2018.
- ↑ Hawaii Bans Certain Sunscreens , aerzteblatt, July 26, 2018.
- ^ The Republic of Palau Bans Sunscreen Chemicals to Protect its Coral Reefs and UNESCO World Heritage site - International Coral Reef Initiative. In: icriforum.org. November 4, 2018, accessed February 20, 2020 .
