Oursinite
| Oursinite | |
|---|---|
| two pale yellow tufts of ourinite from the Shinkolobwe Mine , Katanga , Democratic Republic of the Congo | |
| General and classification | |
| other names |
IMA 1982-051 |
| chemical formula | Co, Mg (UO 2 ) 2 (SiO 3 OH) 2 · 6H 2 O |
|
Mineral class (and possibly department) |
Silicates and Germanates |
|
System no. to Strunz and to Dana |
9.AK.10 ( 8th edition : VIII / B.34) 53.03.01.07 |
| Crystallographic Data | |
| Crystal system | orthorhombic |
| Crystal class ; symbol | 2 / m2 / m2 / m |
| Space group | Cm approx |
| Lattice parameters |
a = 7.0494 Å ; b = 17.550 Å; c = 12.734 Å, α = 90.00 °; β = 90.00 °; γ = 90.00 ° |
| Formula units | Z = 4 |
| Physical Properties | |
| Mohs hardness | 3 to 3.5 |
| Density (g / cm 3 ) | 3,674 (calculated) |
| Cleavage | Please complete |
| colour | pale yellow to yellowish white |
| Line color | Please complete |
| transparency | transparent to translucent |
| shine | Glass gloss |
| radioactivity | radioactive |
| Crystal optics | |
| Refractive indices |
n α = 1.624 n β = 1.640 n γ = 1.650 |
| Birefringence | δ = 0.026 |
| Optical character | biaxial negative |
| Axis angle | 2V = 2V = 76 |
| Other properties | |
| Chemical behavior | soluble in acids |
The mineral oursinite is an extremely rare uranium mineral with the chemical composition Co, Mg (UO 2 ) 2 (SiO 3 OH) 2 · 6H 2 O. It crystallizes in the orthorhombic crystal system and develops mostly needle-like, radial pale yellow crystals up to 1 mm Length. Chemically, it is a cobalt - uranyl - silicate with small amounts of magnesium and nickel may be included.
Etymology and history
The mineral was named after its appearance, which looks similar to a sea urchin (from the French word oursin for sea urchin). According to investigations by M. Deliens and P. Piret in 1983, an assignment to the uranophane group was possible, the space groups Aba2 or Abam were suggested, further information on the crystal structure was not possible. It was not until 2006 that K. Kubatko and P. Burns were able to use single crystal diffractometry to clarify the crystal structure of an oursinite sample from the Shinkolobwe Mine (Kasolo Mine) in the Democratic Republic of the Congo. Type material of the mineral is kept in the Royal Museum for Central Africa of the Belgian municipality of Tervuren under catalog number RGM1321.
classification
The outdated 8th edition of the Strunz lists the Oursinite in the "Uranophangruppe" with the system no. VIII / B.34 and the other members Boltwoodit , Cuprosklodowskit , Kasolit , Natroboltwoodit , Sklodowskit , Uranophan and Uranophan-beta .
The 9th, completely revised edition of Strunz lists the oursinite in the section K “Uranyl island and polysilicates with a uranium: silicon ratio of U: Si = 2: 1” in group 9.AK.10 . This group also includes Cuprosklodowskit and Sklodowskit.
The systematics of minerals according to Dana , which is common in the English-speaking world , assigns ourinite to the uranophane group of the island silicates department, but there in the department of " island silicates with SiO 4 groups and other anions as well as complex cations with (UO 2 ) " with the system No. 53.03.01.07 a.
Crystal structure
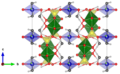
Oursinite crystallizes orthorhombically in the space group Cmce (space group no. 64) with the lattice parameters a = 7.0494 Å , b = 17.550 Å and c = 12.734 Å as well as four formula units per unit cell .
The crystal structure of oursinite primarily corresponds to that of uranophane . It forms layers of uranyl silicate which are held together by the cobalt (Co 2+ ) or magnesium ions (Mg 2+ ). The uranyl units form pentagonal bipyramids, the tips of which are the oxygen atoms of the UO 2 2+ group. In an equatorial position there are five oxygen atoms, which are provided by the silicate groups. In the silicate groups, the silicon is tetrahedrally surrounded by four oxygen atoms, three of which represent the oxide group (O 2- ) and one represents the hydroxide group (OH - ), the hydrogen atom of which forms a hydrogen bond to the opposite uranyl oxygen atom. Additional water molecules contained in the crystal structure bridge one uranyl oxygen atom with one silicate oxygen atom by hydrogen bonding. One of the two uranyl oxygen atoms always remains free and is not coordinated. These layers of uranyl silicate are now held together by cobalt or magnesium ions in such a way that they linearly link the respective OH - groups from two opposing uranyl silicate layers and surround themselves in their equatorial coordination plane with four crystal water molecules , so that an octahedral coordination results for them . A special feature of the structure of oursinite is that the structure contains 80% Co 2+ ions and 20% Mg 2+ ions, and these two types of atoms are statistically distributed over the entire crystal. This is due to the fact that the ion radii of both ions are very similar (Mg 2+ 86 pm, Co 2+ (high spin) 88.5 pm). This is similar, in reverse, to the structure of Sklodovskite , the structure of which is mainly dominated by Mg 2+ ions, but the different arrangement of the silicate tetrahedra leads to a different arrangement of the layers. This suggests that these two structures could form corresponding mixed crystal series , which, however, has not yet been scientifically verified.
properties
The mineral is radioactive due to its uranium content of up to 54.61% . Taking into account the proportions of the radioactive elements in the idealized empirical formula and the Folgezerfälle of the natural decay chains a specific activity of about 97.747 k for the mineral Bq stated / g (compared to natural potassium 0.0312 kBq / g). The quoted value can vary significantly depending on the mineral content and the composition of the levels; selective enrichment or depletion of the radioactive decay products is also possible and changes the activity.
Education and Locations
Oursinite is a rare transformation product that forms in the oxidation zone of primary uranium ore deposits. Depending on where it was found , the mineral is associated with Sklodovskite , Curite , Becquerelite , Schoepite , Kasolit , Soddyite , Lepersonnite , Bijvoetite and Torbernite . So far (as of 2016) the mineral could only be detected at its type locality , the Shinkolobwe Mine (Katanga) in the Democratic Republic of the Congo.
Precautions
Due to the toxicity and radioactivity of the mineral, mineral samples of oursinite should only be kept in dust-tight and radiation-proof containers, but especially never in living rooms, bedrooms or work rooms. Absorption into the body (incorporation, ingestion ) should also be prevented in any case and, for safety, direct body contact should be avoided and respiratory protection mask and gloves should be worn when handling the mineral .
See also
literature
- Karrie-Ann Kubatko and Peter C. Burns: A novel arrangement of silicate tetrahedra in the uranyl silicate sheet of oursinite, In: The American Mineralogist 2006, pp. 333-336. (English, PDF, 530 kB)
Web links
- Mineral Atlas: Oursinite (Wiki)
Individual evidence
- ↑ a b c d e Karrie-Ann Kubatko and Peter C. Burns: A novel arrangement of silicate tetrahedra in the uranyl silicate sheet of oursinite, In: The American Mineralogist 2006, pp. 333–336. (English, PDF, 530 kB) .
- ↑ a b c Webmineral - Oursinite (English).
- ↑ a b c d e Mindat - Oursinite (English).
- ↑ The former name of this group of rooms was Ccma .