Pomarose
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
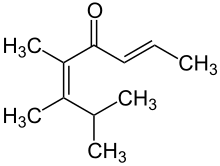
|
|||||||||||||
| General | |||||||||||||
| Surname | Pomarose | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 11 H 18 O | ||||||||||||
| Brief description |
colorless liquid with an intense, fruity-rosy odor reminiscent of apple pie, plums and dried fruits |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 166.26 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Pomarose is a highly intense captive fragrance patented by Givaudan . Pomarose, a doubly unsaturated ketone that does not occur naturally, has a very strong, fruity rose odor with hints of apples , plums and raisins , which is almost exclusively due to the (2 E , 5 Z ) stereoisomer. The (2 E , 5 E ) -configured isomer , on the other hand, is odorless to most people. Even the slightest traces of acid are sufficient for the cis-trans equilibration of the C-5 double bond , which can be observed when standing in glass vessels for a long time.
Discovery and synthesis
When analyzing NMR spectra, Philip Kraft and co-workers initially believed 5,6,7-trimethylocta-2,5-dien-4-one to be an unknown trace component with a damascone odor in a complex reaction mixture. However, the said trace component of this mixture soon turned out to be the constitutionally isomeric 2-methyl-3-isopropylhepta-2,5-dien-4-one, which in fact also has a damascone odor. Out of sheer curiosities in the structure-odor relationships of this compound, 5,6,7-trimethylocta-2,5-dien-4-one (Pomarose) was then also synthesized, and surprisingly turned out to be a perfect perfumery hit with radiant fruity rose flowers. Note that also reminded of apple pie, plums and dried fruits. In addition, it had a low odor threshold of only 0.5 ng / l air. The synthesis consisted of boron trifluoride -catalyzed addition of methylisopropyl ketone to 1-ethoxyprop-1-yne to give 2,3,4-trimethylpent-2-enoic acid ethyl ester, which was then carried out in situ by a Grignard reaction with propen-1-ylmagnesium bromide -Enolization has been transferred to the target molecule.
Use in perfumery
Pomarose made its debut with 0.36% in “ Be Delicious for Men ” by Olivier Gillotin and Pierre Negrin. The DKNY perfume letter was looking for a fragrance for 'a determined-looking, urbane man who is open to everything that life in the Big Apple of New York has to offer'. In this context, Pomarose's apple and rose theme fitted perfectly so the perfume was created around this fragrance. Only a year later, pomarose was 0.43% in " Unforgivable " ( Sean John , 2006) in a champagne overdosed chord by David Apel, Pierre Negrin, Caroline Sabas and Aurélien Guichard. In “1 Million” ( Paco Rabanne , 2008) Christophe Raynaud, Olivier Pescheux and Michel Girard contrast 0.18% of this extremely intense fragrance with a light leather note. Ellen Molner and Rodrigo Flores-Roux used pomarose in the aromatic Fougere -Parfüm " CK free " ( Calvin Klein , 2009), and Olivier Pescheux contrasted the oak moss -Riechstoff Evernyl with pomarose in "Legend" (Mont Blanc, 2011). In women's perfumery, Pomarose was used by David Apel in the Piña Colada chord of " Unforgivable Woman " (Sean John, 2006), and it can also be found as part of the ' hairspray ' rose note by "John Galliano" ( John Galliano , 2008).
Related fragrances
- α- and β-damascone
- Damascenone
Individual evidence
- ↑ Günther Ohloff, Wilhelm Pickenhagen, Philip Kraft: Scent and Chemistry - The Molecular World of Odors . Helvetica Chimica Acta, Zurich 2012, ISBN 978-3-906390-66-6 , p. 82 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Patent EP1149820 : 2-, 5-, 6-, 7-, 8-substituted oct-2-ene-4-ones. Registered on April 21, 2001 , inventor: Philip Kraft.
- ^ Karl A. D. Swift, Royal Society of Chemistry (Great Britain): Advances in flavors and fragrances: from the sensation to the synthesis . Royal Society of Chemistry, Cambridge 2002, ISBN 0-85404-821-9 , pp. 138 ( limited preview in Google Book search).
- ↑ Philip Kraft, Caroline Denis, Walter Eichenberger: 5,6,7-Trimethylocta-2,5-dien-4-one - A Suspected Odorant with Surprising Olfactory Properties . In: European Journal of Organic Chemistry . 2001, No. 12, 2001, pp. 2363-2369. doi : 10.1002 / 1099-0690 (200106) 2001: 12 <2363 :: AID-EJOC2363> 3.0.CO; 2-E .
- ↑ Günther Ohloff, Wilhelm Pickenhagen, Philip Kraft: Scent and Chemistry - The Molecular World of Odors . Helvetica Chimica Acta, Zurich 2012, ISBN 978-3-906390-66-6 , p. 217 ( limited preview in Google Book search).